Applications of Hess’s Law (AQA AS Chemistry) : Revision Note
Did this video help you?
Hess's Law Calculations
You must make sure that you can apply Hess' Law effectively and calculate enthalpy changes in different situations
Remember - it is the data that is important
Check whether the data you have been given is formation data or combustion data, and then complete the cycle or calculation according to that
Worked Example
Calculating the enthalpy change of formation of ethane
Calculate ΔHf [ethane].
The relevant change in standard enthalpy of combustion (ΔHc) values are shown in the table below:

Answer
Step 1: Write the equation for enthalpy change of formation at the top and add oxygen on both sides
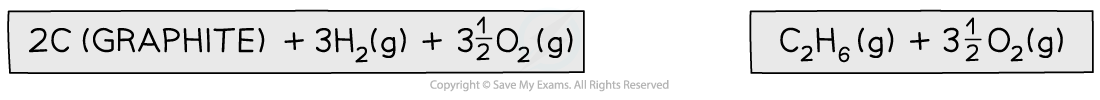
Step 2: Draw the cycle with the combustion products at the bottom
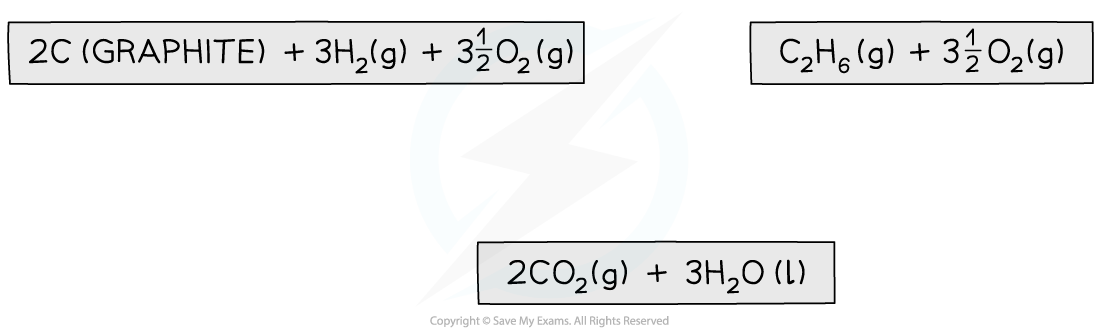
Step 3: Draw all arrows in the correct direction
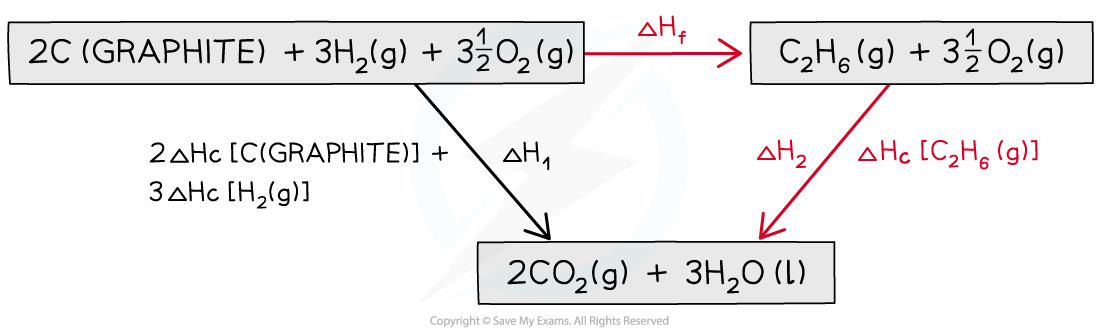
Step 4: Apply Hess’s Law

Calculating average bond energies using Hess’s cycles
Bond energies cannot be found directly so enthalpy cycles are used to find the average bond energy
This can be done using enthalpy changes of atomisation and combustion or formation
The enthalpy change of atomisation (ΔHatꝋ ) is the enthalpy change when one mole of gaseous atoms is formed from its elements under standard conditions.
Eg. ΔHatꝋ [H2] relates to the equation:
½ H2(g) → H(g)
Worked Example
Calculating average C-H bond energy
Calculate the average bond energy of the C-H bond using the relevant ΔHfꝋ and ΔHatꝋ values in the table below:

Answer
Step 1: Write down the equation for the dissociation of methane at the top

Step 2: Write down the elements at the bottom
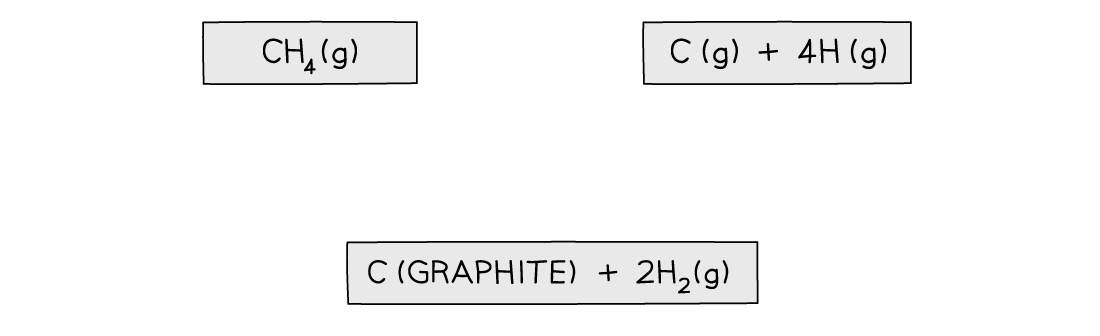
Step 3: Draw all arrows in the correct direction
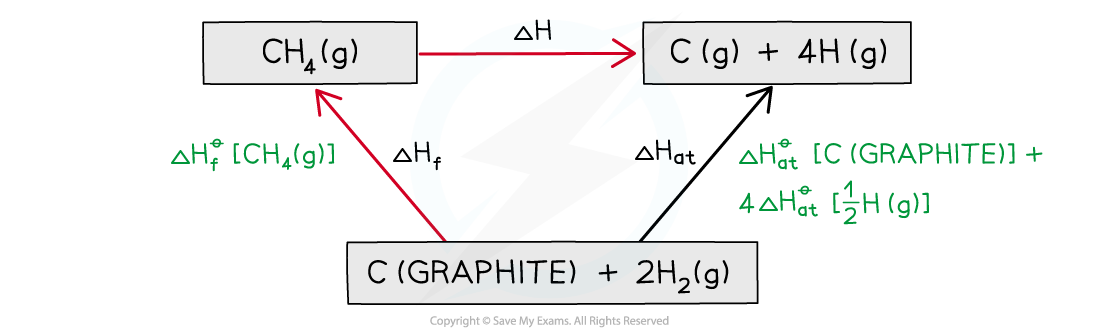
Step 4: Apply Hess’s Law

Step 5: Since there are 4 C-H bonds in methane:

Examiner Tips and Tricks
Remember: Take into account the number of moles of each reactant and product.
For example, there are two moles of NaHCO3(s) so the ΔHf value is multiplied by 2.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
