Rate: Inhibitor Concentration (Cambridge (CIE) AS Biology): Revision Note
Exam code: 9700
Rate: inhibitor concentration
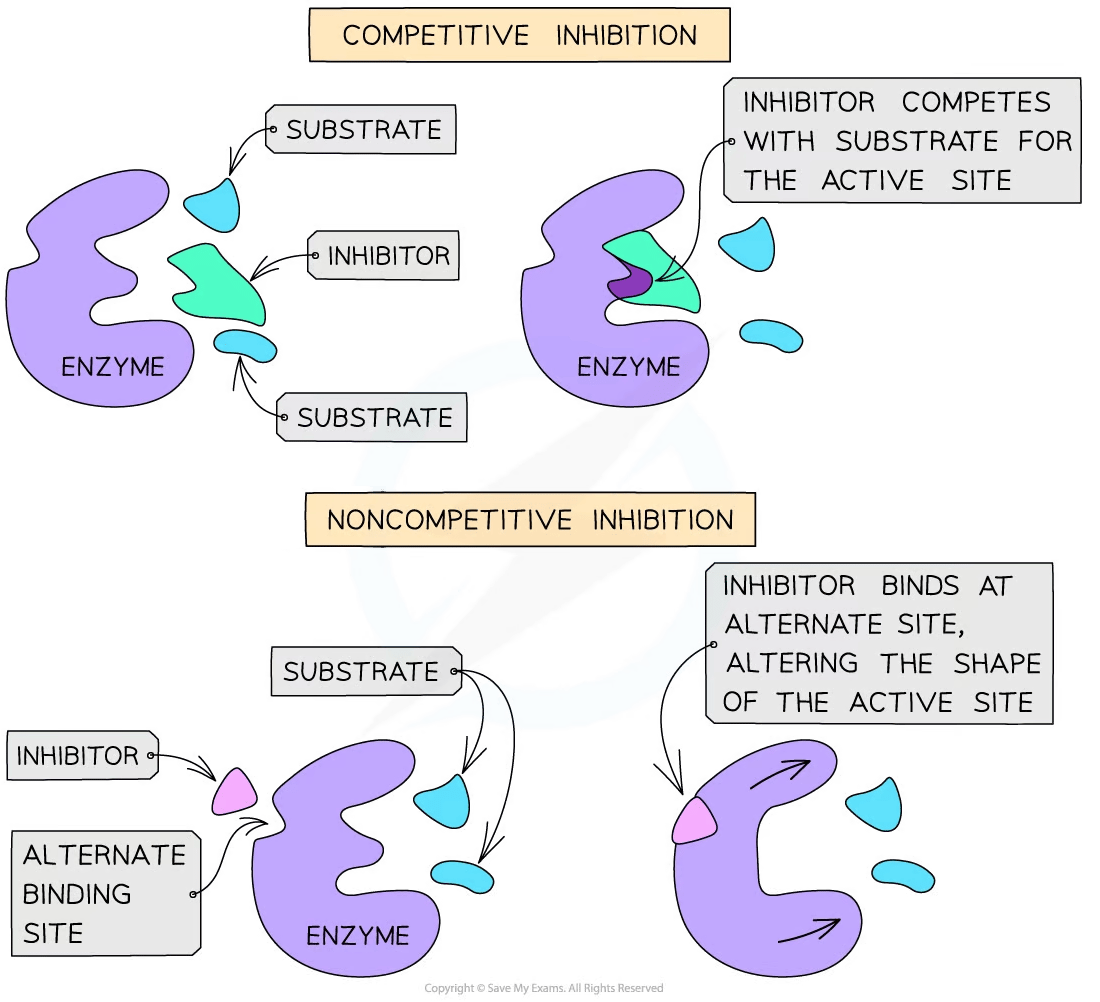
There are two types of inhibitors:
Competitive inhibitors have a similar shape to that of the substrate molecules
This means they compete with the substrate for the active site
Non-competitive inhibitors bind to the enzyme at an alternative site, altering the shape of the active site
This prevents the substrate from binding to it
Both types of inhibitors slow down or stop enzyme activity
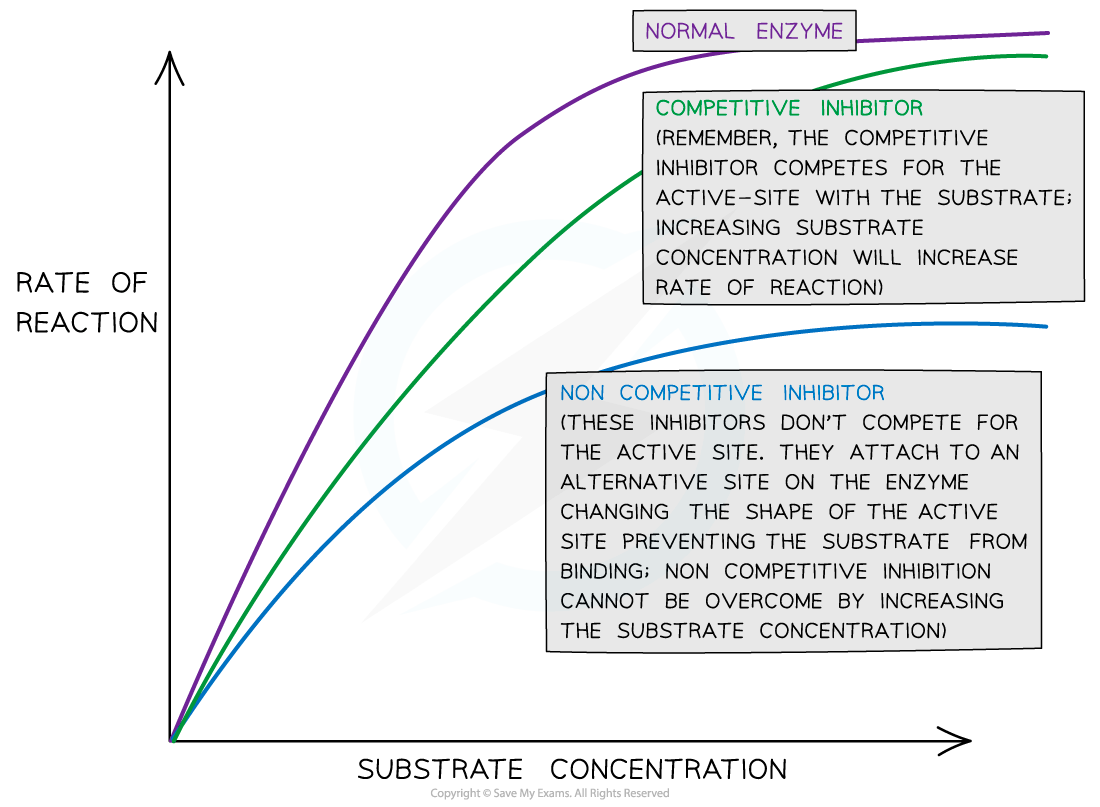
Increasing the concentration of an inhibitor reduces the rate of reaction
Eventually, if inhibitor concentration continues to be increased, the reaction will stop completely
For competitive inhibitors, countering the increase in inhibitor concentration by increasing the substrate concentration can increase the rate of reaction once more
This is because more substrate molecules mean they are more likely to collide with enzymes and form enzyme-substrate complexes before the inhibitor can bind
For non-competitive inhibitors, increasing the substrate concentration cannot increase the rate of reaction once more
This is because the shape of the active site of the enzyme remains changed, so enzyme-substrate complexes are still unable to form despite the high number of substrate molecules present
Examiner Tips and Tricks
While a competitive inhibitor will lower the initial rate of reaction (by occupying some of the available active sites), eventually the same amount of product will be produced as would have been produced without the competitive inhibitor (the maximal rate is not affected).
Non-competitive inhibitors lower the initial rate of reaction and the maximal rate of reaction (a lower amount of product is produced than would normally be produced).

Unlock more, it's free!
Was this revision note helpful?
