Reducing & Non-Reducing Sugars (Cambridge (CIE) AS Biology): Revision Note
Exam code: 9700
Reducing & non-reducing sugars
Sugars can be classified as reducing or non-reducing; this classification is dependent on their ability to donate electrons
Reducing sugars can donate electrons (the carbonyl group becomes oxidised), the sugars become the reducing agent
Thus reducing sugars can be detected using the Benedict’s test as they reduce the soluble copper sulphate to insoluble brick-red copper oxide
Examples: glucose, fructose, maltose
Non-reducing sugars cannot donate electrons, therefore they cannot be oxidised
To be detected non-reducing sugars must first be hydrolysed to break the disaccharide into its two monosaccharides before a Benedict’s test can be carried out
Example: sucrose
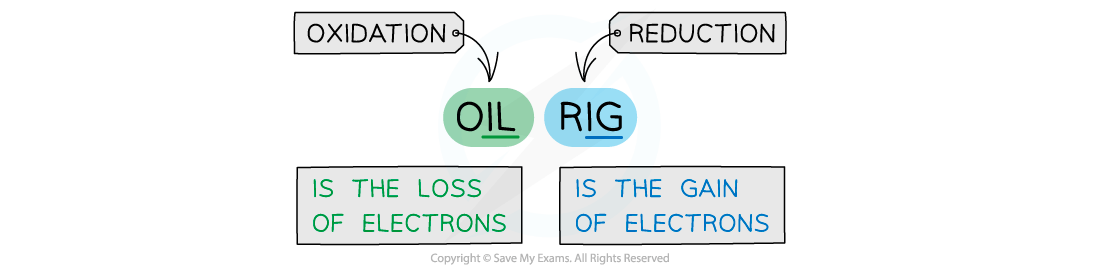
Examiner Tips and Tricks
Become familiar with the OILRIG mnemonic to remember what happens to a molecule when electrons are lost from it (oxidation) or gained by it (reduction).

Unlock more, it's free!
Was this revision note helpful?
