Practical Skill: Investing Factors Affecting Enzyme Reaction Rates
- When an enzyme is kept in conditions that are outside its optimum pH the shape of its active site may change
- As a result, extreme pH concentrations can disrupt the ability of an enzyme to bind with its substrate and reduce enzyme reaction rates
- Experiments can be carried out to determine the effect of changing pH on the rate of a reaction catalysed by a digestive enzyme
Effect of pH on enzyme reaction rates
- Use the enzyme amylase to breakdown starch at a range of pH values, using iodine solution as an indicator for the reaction occurring
- Amylase is an enzyme that digests starch (a polysaccharide of glucose) into maltose (a disaccharide of glucose)
- Use a continuous sampling technique to monitor the progress of the reaction
- Starch can be tested for easily using iodine solution

Iodine can be used qualitatively to indicate the presence or absence of starch from a sample
Apparatus
- Test tubes
- Buffer solutions at different pH levels
- Amylase solution
- Iodine solution
- Starch solution
- Pipettes
- Spotting tile
- Timer
- Gloves
- Goggles
Method
- Wear goggles and gloves
- Enzymes have the potential to cause allergic reactions if they come into direct contact with skin
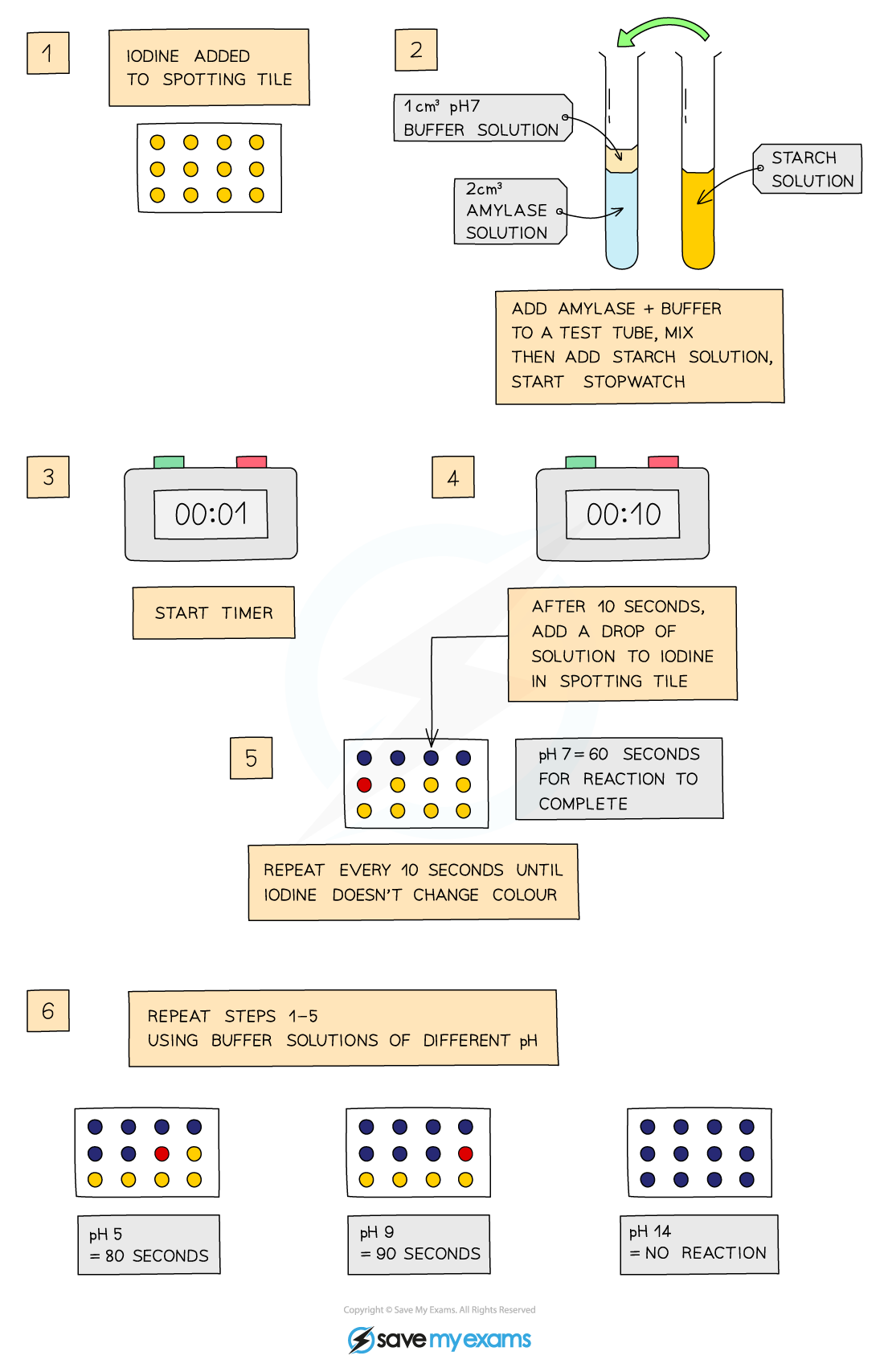
- Place single drops of iodine solution in rows on the tile
- Iodine solution is orange-brown
- Label a test tube with the pH to be tested
- Use the syringe to place 2cm3 of amylase in the test tube
- Equal volume and concentration of enzyme should be used so these variables are controlled and the effect of changing pH can be measured
- Add 1cm3 of buffer solution to the test tube using a syringe
- Use another test tube to add 2cm3 of starch solution to the amylase and buffer solution, start the stopwatch whilst mixing using a pipette
- Equal volume and concentration of the substrate (starch) should be used so these variables are controlled and the effect of changing pH can be measured
- Mixing enables the enzymes and substrate to be equally mixed
- After 10 seconds, use a pipette to place one drop of the mixture on the first drop of iodine, which should turn blue-black
- This indicates starch is still present
- Wait another 10 seconds and place another drop of the mixture on the second drop of iodine
- Repeat every 10 seconds until iodine solution remains orange-brown
- This means amylase has broken down all of the starch so nothing is left to react with the iodine
- Repeat experiment at different pH values
- The less time the iodine solution takes to remain orange-brown, the quicker all the starch has been digested and so the better the enzyme works at that pH
Investigating the effect of pH on enzyme activity
Limitations
- The above method can be adapted to control temperature by using a water bath at 35℃
- All solutions that need to be used (starch, amylase, pH buffers) should be placed in a water bath and allowed to reach the temperature (using a thermometer to check) before being used
- A colorimeter can be used to measure the progress of the reaction more accurately; with a solution containing starch being darker and glucose lighter (as a result of the colour-change of iodine) – this will affect the absorbance or transmission of light in a colorimeter
Effect of bile salts on enzyme reaction rates
- Bile salts aid the process of emulsification
- They bind to large fat droplets and break them down into smaller fat droplets
- Lipase can only act on the surface of the fat droplets so emulsification increases the surface area for lipase to act on, increase the rate of enzyme reaction
- Experiments can be conducted to investigate the effect of bile salts on the rate of enzyme reactions
Apparatus
- Gloves
- Goggles
- Bile salts solution of a known concentration
- Lipase solution of a known concentration
- Lipid solution of a known concentration
- pH monitor
- Beakers
- Test tubes
Method
- An experiment may investigate the effect of the absence or presence of bile salts
- Lipase solution is mixed with lipids and bile salts and the rate of reaction is measured. Lipase solution is mixed with lipids only and the rate of reaction is measured. The rates of reaction can be compared
- The rate of reaction can be measured by measuring the pH level (the dependent variable) as lipid digestion involves the release of fatty acids which reduce pH
- When the pH levels-off the digestion of the substrate is complete
- The experiment could also be carried out using different concentrations of bile salts (the independent variable), measuring the rate of reaction at each concentration
- E.g. 2%, 3% and 4% bile salts solutions added to lipase
- If there is too high or too low a concentration of bile salts in the lumen of the gut then lipid digestion will be incomplete. This can cause indigestion and diarrhoea
Examiner Tip
Make sure that all the other variables in the experiment are controlled. Temperature and the concentration of the substrate and enzymes must be the same when each repeat of the experiment begins.

