Fluid Properties (College Board AP® Physics 1: Algebra-Based): Study Guide
Fluid properties
What is a fluid?
Liquids and gases are collectively known as fluids
Unlike solids, these have weak interactions between particles
In a liquid, particles are weakly bonded, allowing the substance to flow
In a gas, particles are not bound together, so they can spread out freely
Fluids have no fixed shape
Liquids fill the base of a container
Gases expand to fill a container completely
Most questions will assume that a fluid is ideal
An ideal fluid is incompressible
This means that force applied to one region of a fluid is exerted on other regions of the fluid
An ideal fluid has a viscosity of zero
This means resistive forces do not need to be considered in questions involving flowing ideal fluids
Fluid density
What is density?
Density is defined as:
The mass per unit volume of a substance
The density of an ideal fluid can be a characteristic property
This is because an ideal fluid is incompressible, so the density remains constant
Density equation
The density of a fluid can be calculated using:
Where:
= density of the fluid, measured in
= mass of the fluid, measured in
= volume of the fluid, measured in
This equation appears on the equation sheet
Measuring density
Measuring the density of regular objects
The volume of a regular object can be calculated accurately by taking measurements of its dimensions
Common examples are cubes, cylinders, and spheres
A procedure to find the volume of a regular object is as follows:
Place the object on a digital balance and note down its mass
Use either a ruler, callipers, or a micrometer to measure the object’s dimensions (width, height, length, radius) – the measuring apparatus will depend on the size of the object
Repeat these measurements and take an average of these readings
Use these average values and a suitable formula for the regular shape to calculate its volume
Substitute the mass and volume into the density equation
Measuring the density of irregular objects
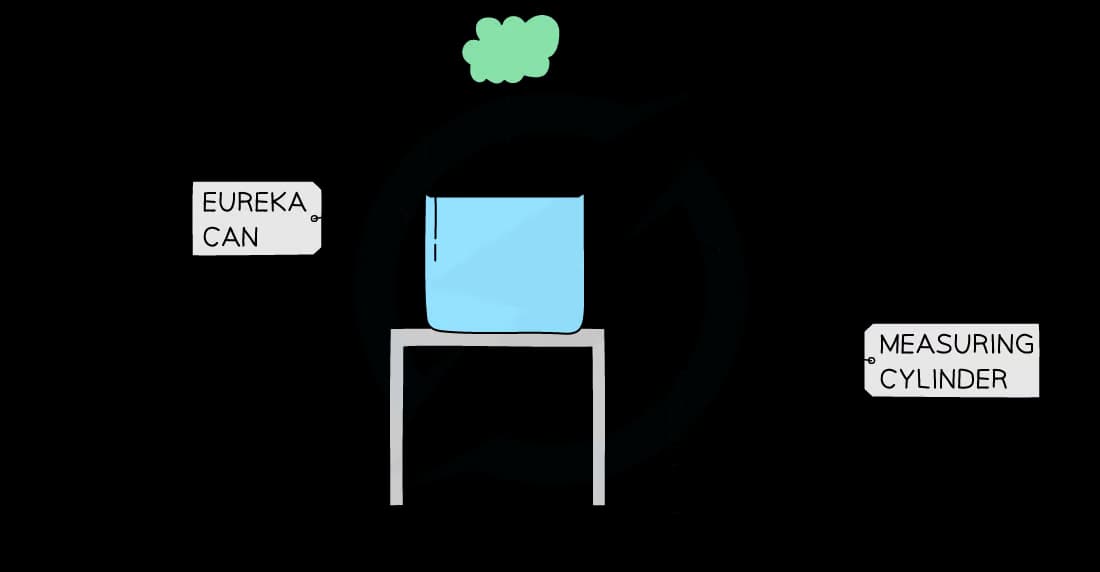
The density of an irregularly shaped object can be found using the displacement method
This method requires the object to displace a volume of water
A beaker with a spout pointing downwards, called a displacement (or eureka) can, is required
A procedure to find the volume of an irregular object is as follows:
Place the object on a digital balance and note down its mass
Fill the displacement can with water up to a point just below the spout
Place an empty measuring cylinder below its spout
Carefully lower the object into the eureka can
Measure the volume of the displaced water in the measuring cylinder
The volume of water displaced is equal to the volume of the object
Repeat these measurements and take an average before calculating the density
Equipment to measure the density of irregular objects

Unlock more, it's free!
Did this page help you?