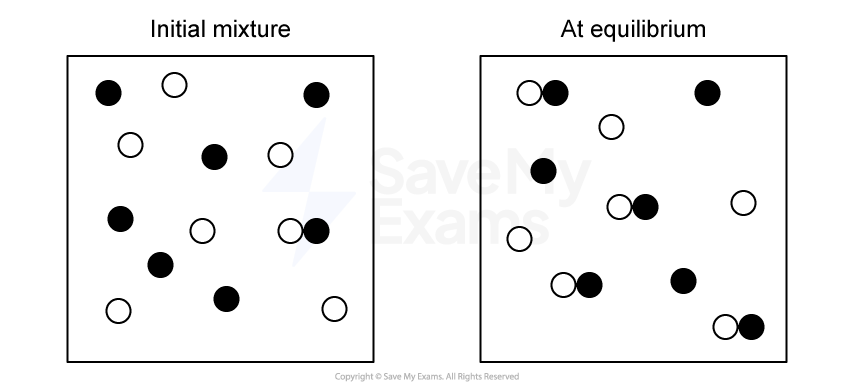
A reversible reaction is shown below:
X2 (g) ⇌ 2X (g)
For this reaction, Kc = 0.80 at a certain temperature.
A mixture is prepared with the following initial concentrations:
[X2] = 0.20 M
[X] = 0.60 M
Write the expression for the reaction quotient Qc.
Calculate the value of Qc using the initial concentrations.
State whether the reaction will shift toward reactants or products as it moves toward equilibrium. Explain your answer using the relationship between Qc and Kc.
State whether the equilibrium concentration of X be greater than, equal to, or less than its initial value.
Did this page help you?