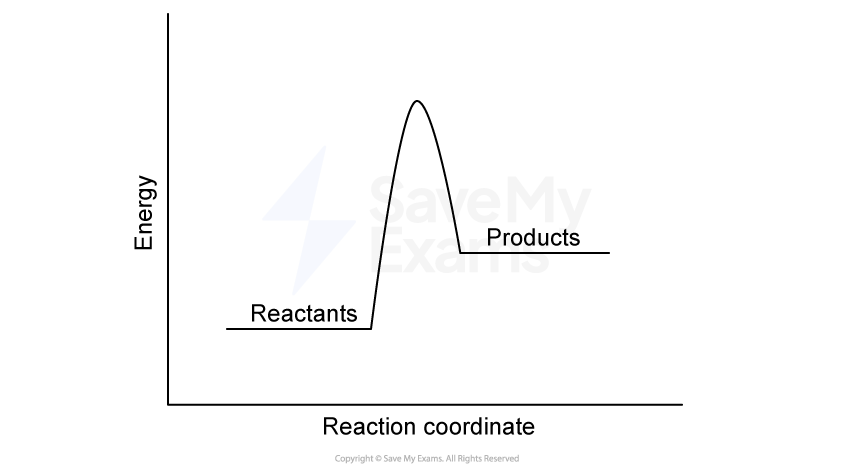
A student investigates the combustion of methane, CH4, in oxygen under standard conditions. The reaction is represented below:
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l)ΔH∘=−890 kJ/mol
Explain the meaning of ΔH∘ = −890 kJ/mol.
Calculate the total energy released when 3.00 mol of CH4 combusts.
Methane combustion is exothermic. Explain this in terms of bond enthalpies, referencing the bonds broken in the reactants and the bonds formed in the products.
Did this page help you?