Thermodynamic Favorability (College Board AP® Chemistry): Study Guide
Thermodynamic Favorability
Gibbs free energy provides an effective way of focusing on a reaction system at constant temperature and pressure to determine whether the reaction is thermodynamically favorable
A thermodynamically favorable reaction is one that occurs without the need for additional energy input after the reactants are mixed
A thermodynamically favored reaction will have a negative ΔGo value
For a reaction to be spontaneous, Gibbs free energy must be a negative value (ΔGo ≤ 0)
Examiner Tips and Tricks
Historically, a thermodynamically favorable reaction has been referred to as a spontaneous reaction.
Thermodynamically favored is the preferred term as it avoids the common misunderstanding that the reaction happens suddenly or without cause.
Thermodynamic Favorability & Temperature Changes
The temperature conditions for a process to be thermodynamically favored (whereby ΔGo is negative and less than 0) can be predicted from the signs of ΔHo and ΔSo

If ΔHo is negative (exothermic reaction) and ΔSo is positive:
This will result in a negative ΔGo
The reaction is always thermodynamically favored
No calculation of ΔGo would be necessary to determine this
If ΔHo is negative (exothermic reaction) and ΔSo is negative:
At high temperatures -TΔSo will be very large and positive and will overcome ΔHo
At high temperatures ΔGo is positive
The reaction is thermodynamically favored at low temperatures
ΔGo and Exothermic Reactions
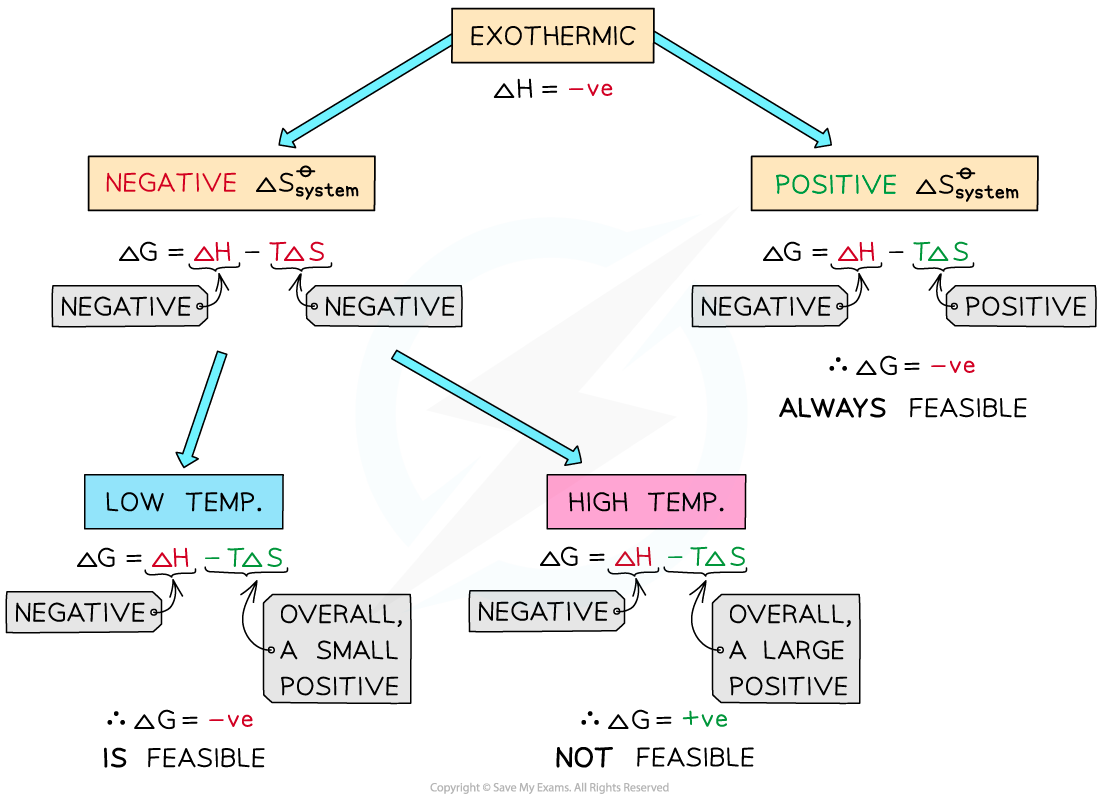
If ΔHo is negative and If ΔSo is negative the reaction will only be thermodynamically favored at low temperatures
If ΔHo is positive (endothermic reaction) and ΔSo is negative:
Both ΔHo and -TΔSo will be positive
This results in a positive ΔGo
Therefore, regardless of the temperature, endothermic with a negative ΔSo is not thermodynamically favored
No calculation of ΔGo is necessary to determine that the process is thermodynamically unfavored
If ΔHo is positive (endothermic reaction) and ΔSo is positive
ΔHo is positive and -TΔSo will be negative
At low temperatures -TΔSo cannot overcome the larger ΔHo
At low temperatures, ΔGo is positive and the reaction is thermodynamically favored
At higher temperatures, the second term will become negative enough to overcome the ΔH resulting in a negative ΔGo
ΔGo and Endothermic Reactions
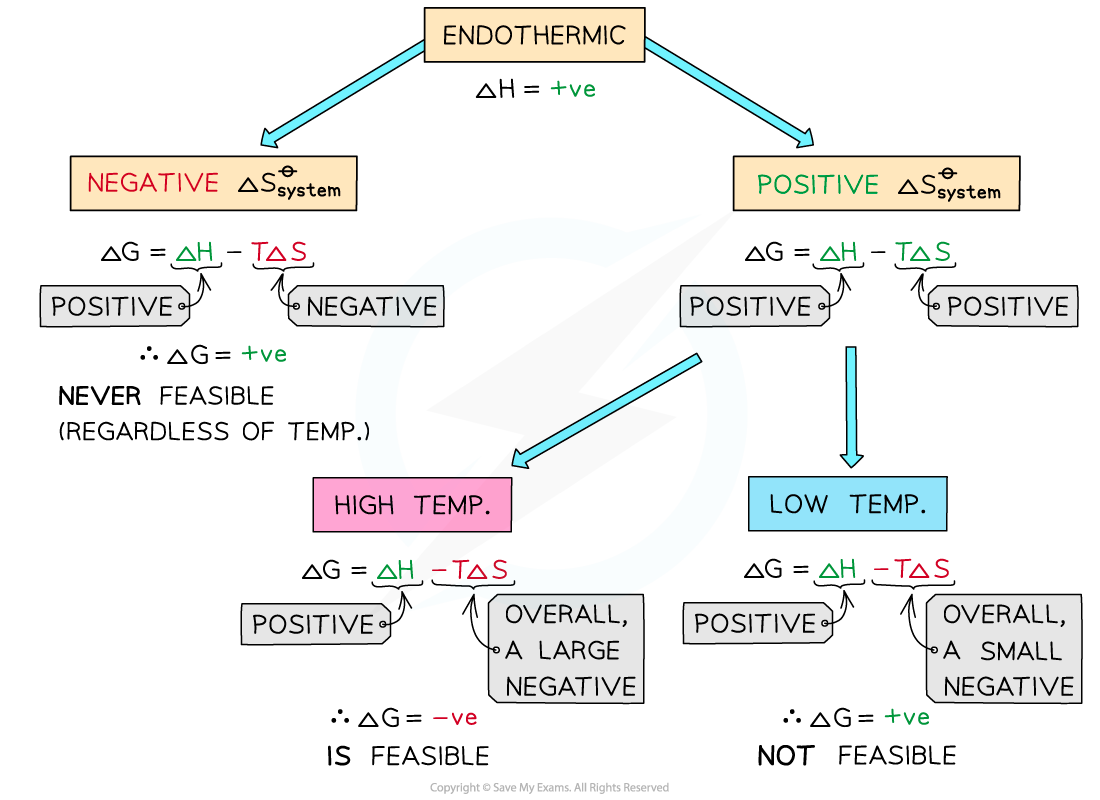
If ΔHo is positive and If ΔSo is positive the reaction will only be thermodynamically favored at a high temperatures
Summary table showing the relationship between ΔHo , ΔSo and ΔGo
ΔHo | ΔSo | ΔGo < 0 favoured at: | Reaction is: |
|---|---|---|---|
< 0 | > 0 | all temperatures | thermodynamically favored |
> 0 | < 0 | no temperatures | thermodynamically unfavored |
> 0 | > 0 | high temperatures | thermodynamically favoured as temperature increases |
< 0 | < 0 | low temperatures | thermodynamically unfavored as temperature increases |
Worked Example
For a reaction with the following enthalpy and entropy changes:
ΔSo = -75.8 kJ mol-1
ΔHo = -13.65 kJ mol-1
a) Calculate ΔGo at 298 K.
b) Calculate the temperature the reaction will become thermodynamically favored.
Answer:
a) At 298 K:
Convert ΔSo from J K-1 mol-1 into kJ K-1 mol-1
=
= – 0.0758 kJ K-1 mol-1
ΔGo= ΔHo – TΔSo
ΔGo = -13.65 – 298 x (–0.0758)
ΔGo= + 8.94 kJ mol-1
b) The temperature at which the reaction will become thermodynamically favored:
Rearrange ΔGo= ΔHo – TΔSo to find T
When ΔGo= 0, then
T=
When ΔHo and ΔSo are both negative, decreasing the temperature causes reactions to be thermodynamically favored
Therefore below 180 K, the reaction is thermodynamically favored, and above 180 K the reaction is not thermodynamically favored

Unlock more, it's free!
Did this page help you?