The Henderson-Hasselbalch Equation (College Board AP® Chemistry): Study Guide
The Henderson-Hasselbalch Equation
The pH of a buffer solution can be calculated using:
The Ka of the weak acid
The equilibrium concentration of the weak acid and its conjugate base (salt)
To determine the pH, the concentration of hydrogen ions is needed which can be found using the equilibrium expression
Ka =
which can be rearranged to [H+] = Ka x
To simplify the calculations, logarithms are used such that the expression becomes:
-log10[H+] = -log10 Ka x -log10
Since -log10 [H+] = pH, the expression can also be rewritten as:
pH = pKa + log10
This is known as the Henderson-Hasselbalch equation
For weak bases a similar derivation occurs:
pOH = pKb + log10
Worked Example
Calculate the pH of a buffer solution containing 0.305 mol dm-3 of ethanoic acid and 0.520 mol dm-3 sodium ethanoate.
(pKa of ethanoic acid = 4.76)
Answer:
Step 1: Write down Hendersen-Hasselback equation
pH = pKa + log10
Step 2: Substitute in values
pH = 4.76 + log10
pH = 4.99 (given to 2 d.p)
Worked Example
Calculate the pH of a buffer solution containing 0.0400 mol dm-3 of ethylamine and 0.0865 mol dm-3 ethyl ammonium chloride.
(pKb of ethylamine = 3.99)
Answer:
Step 1: Write down Hendersen-Hasselback equation
pOH = pKb + log10
Step 2: Substitute in values
pOH = 3.99 + log10
= 4.32
pOH = 4.32 therefore pH = 9.68 (given to 2 d.p)
What happens when acids and bases are added to a buffer solution?
Consider ethanoic acid and sodium enthanoate as a buffer system
CH3COOH (aq) CH3COO– (aq) + H+ (aq)
When H+ ions are added to an acidic buffer:
The equilibrium position shifts to the left as H+ ions react with CH3COO- ions to form more CH3COOH until equilibrium is re-established
As there is a large reserve supply of CH3COO- the concentration of CH3COO- in solution doesn’t change much as it reacts with the added H+ ions
As there is a large reserve supply of CH3COOH the concentration of CH3COOH in solution doesn’t change much as CH3COOH is formed from the reaction of CH3COO- with H+
The ratio of
remains reasonably constant and thus does not significantly change the solution pH

When hydrogen ions are added to the solution the pH of the solution would decrease. However, the ethanoate ions in the buffer solution react with the hydrogen ions to prevent this and keep the pH constant
When OH- ions are added to an acidic buffer:
The OH- reacts with H+ to form water
OH- (aq) + H+ (aq) → H2O (l)
The H+ concentration decreases
The equilibrium position shifts to the right and more CH3COOH molecules ionise to form more H+and CH3COO- until equilibrium is re-established
CH3COOH (aq) → H+ (aq) + CH3COO- (aq)
As there is a large reserve supply of CH3COOH the concentration of CH3COOH in solution doesn’t change much when CH3COOH dissociates to form more H+ ions
As there is a large reserve supply of CH3COO- the concentration of CH3COO- in solution doesn’t change much
The ratio of
remains reasonably constant and thus does not significantly change the solution pH
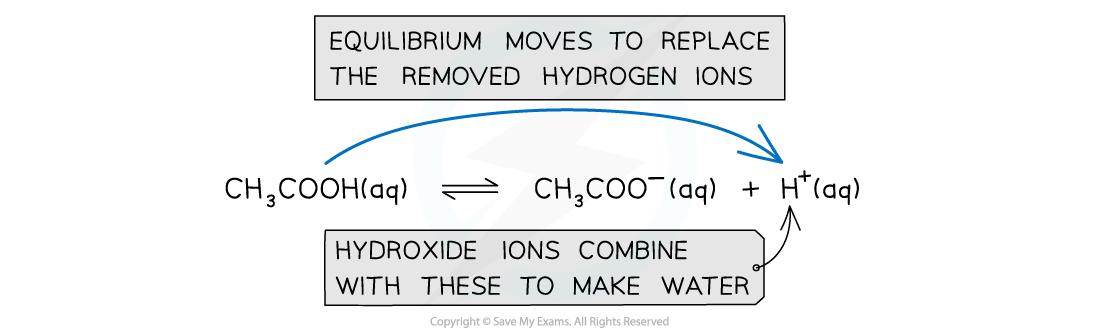
When hydroxide ions are added to the solution, the hydrogen ions react with them to form water. The decrease in hydrogen ions would mean that the pH would increase however the equilibrium moves to the right to replace the removed hydrogen ions and keep the pH constant
Changing concentrations of HA and A-
The Hendersen-Hasselback equation can be used to demonstrate the implications on the pH of the buffer solution is the concentration on HA and A– are changed
pH = pKa + log10
For example,
A common buffer contains CH3COOH and CH3COO–
If the ratio [CH3COOH] and [CH3COO–] change the pH of the buffer solution will change
Ratio of [CH3COOH] and [CH3COO–] in a buffer
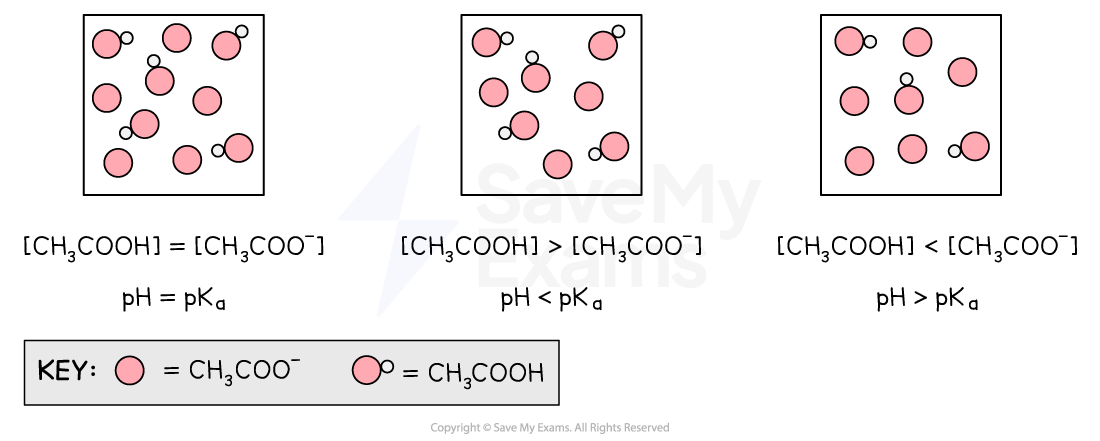
The pH of a buffer will vary depending on the concentration of the acid and salt
pH = pKa + log10
If [CH3COOH] and [CH3COO–] are equal:
The pH will = pKa
If [CH3COOH] is greater than (>) [CH3COO–]
The pH will be greater than pKa
If [CH3COOH] is less than (<) [CH3COO–]
The pH will be less than pKa

Unlock more, it's free!
Did this page help you?