Solubility-Product Constant (College Board AP® Chemistry): Study Guide
Solubility-Product Constant, Ksp
Solubility is defined as the number of grams or moles of compound needed to saturate 100 mL of water, or it can also be defined in terms of 1 litre of water, at a given temperature
For example, sodium chloride (NaCl) is considered to be a soluble salt as a saturated solution contains 36 g of NaCl per 100 mL of water
Lead chloride (PbCl2) on the other hand is an insoluble salt as a saturated solution only contains 0.99 g of PbCl2 per 100 mL of water
Solubility product
The solubility product (Ksp) is:
The product of the concentrations of each ion in a saturated solution of a relatively soluble salt
At 298 K
Raised to the power of their relative concentrations
C (s) ⇌ aAx+ (aq) + bBy- (aq)
Ksp = [Ax+ (aq)]a [By- (aq)]b
When an undissolved ionic compound is in contact with a saturated solution of its ions, an equilibrium is established
The ions move from the solid to the saturated solution at the same rate as they move from the solution to the solid
For example, the undissolved magnesium chloride (MgCl2) is in equilibrium with a saturated solution of its ions
MgCl2 (s) ⇌ Mg2+ (aq) + 2Cl- (aq)
Ions in a saturated solution
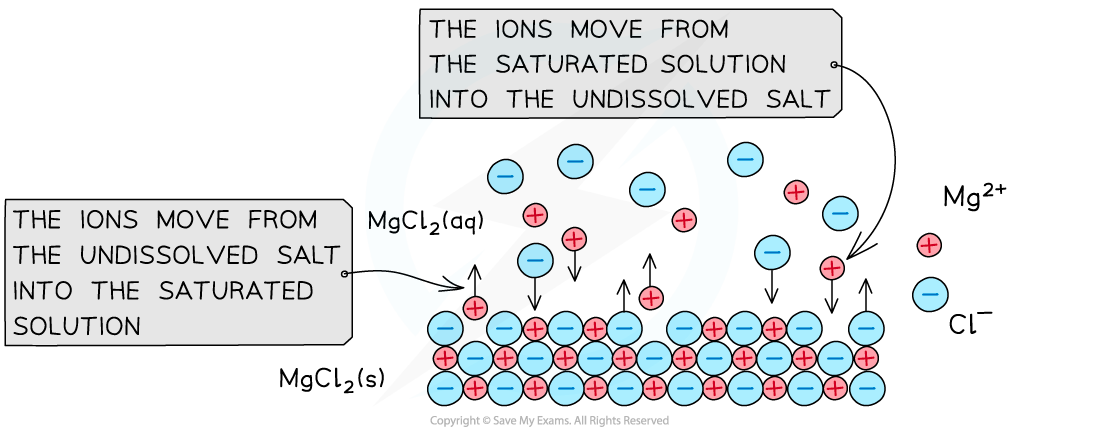
When the undissolved MgCl2 salt comes in contact with its ions in a saturated solution, an equilibrium between the salt and ions is established
The solubility product for this equilibrium is:
Ksp = [Mg2+ (aq)] [Cl- (aq)]2
The Ksp is only useful for sparingly soluble salts
The smaller the value of Ksp, the lower the solubility of the salt
Solubility-Product Constant Calculations
Calculations involving the solubility product (Ksp) are solved as other equilibrium problems
Worked Example
Calculating the solubility product of a compound from its solubility
Calculate the solubility product of a saturated solution of lead(II) bromide, PbBr2, with a solubility of 1.39 x 10-3 mol dm-3.
Answer:
Step 1: Write down the equilibrium equation and expression:
PbBr2 (s) ⇌ Pb2+ (aq) + 2Br- (aq)
Ksp = [Pb2+(aq)] [Br- (aq)]2
Step 2: Set up an equilibrium table:
Reaction | PbBr2 (s) | ⇌ | Pb2+ (aq) | + | 2Br- (aq) |
Initial Conc. | Solid |
| 0 M |
| 0 M |
Change | –x dissolves |
| + x |
| + 2x |
Equilibrium | solid remaining |
| + x |
| + 2x |
Step 3: Calculate the ion concentrations in the solution:
[PbBr2(s)] = 1.39 x 10-3 mol dm-3
The ratio of PbBr2 to Pb2+ is 1:1
[Pb2+(aq)] = [PbBr2(s)] = 1.39 x 10-3 mol dm-3
The ratio of PbBr2 to Br- is 1:2
[Br-(aq)] = 2 x [PbBr2(s)] = 2 x 1.39 x 10-3 mol dm-3 = 2.78 x 10-3 mol dm-3
Step 4: Substitute the values into the expression to find the solubility product:
Ksp = (1.39 x 10-3) x (2.78 x 10-3)2
Ksp = 1.07 x 10-8
Therefore, the solubility product is 1.07 x 10-8
Worked Example
Calculating the solubility of a compound from its solubility product
Calculate the solubility of a saturated solution of copper(II) oxide, CuO, with a solubility product of 5.9 x 10-36.
Answer:
Step 1: Write down the equilibrium equation and expression:
CuO (s) ⇌ Cu2+ (aq) + O2- (aq)
Ksp = [Cu2+ (aq)] [O2- (aq)]
Step 2: Set up an equilibrium table:
Reaction | CuO (s) | ⇌ | Cu2+ (aq) | + | O2- (aq) |
Initial Conc. | Solid |
| 0 M |
| 0 M |
Change | –x dissolves |
| + x |
| + x |
Equilibrium | solid remaining |
| + x |
| + x |
Step 3: Simplify the equilibrium expression:
The ratio of Cu2+ to O2- is 1:1
[Cu2+(aq)] = [O2-(aq)] so the expression can be simplified to:
Ksp = [Cu2+ (aq)]2
Step 4: Substitute the value of Ksp into the expression to find the concentration:
5.9 x 10-36 = [Cu2+ (aq)]2
[Cu2+ (aq)] =
[Cu2+ (aq)] = 2.4 x 10-18
Since [CuO (s)] = [Cu2+ (aq)], the solubility of copper oxide is 2.4 x 10-18
Examiner Tips and Tricks
Remember that the solubility product is only applicable to very slightly soluble salts and cannot be used for soluble salts such as:
Group 1 element salts
All nitrate salts
All ammonium salts
Many sulfate salts
Many halide salts (except for lead(II) halides and silver halides)
Applying Solubility Rules to Ksp
A salt is soluble if at least 0.1 mol of the salt will dissolve in 1 L of water
Saturated solutions of insoluble salts have concentration that are less than 0.1 molar, however, most insoluble salts do dissolve to a small extent
As we have seen before with equilibria, we can relate Ksp to the reaction quotient, Q
This will help us predict if a precipitation reaction will produce more or less precipitate to adjust to equilibrium
If Q > K the reaction will favour the left hand side (backward reaction) and form more reactant
Solubility decreases as precipitate forms
If Q < K the reaction will favour the right hand side (forward reaction) and form more product
Solubility increases as more ions dissolve
And if Q = K, the reaction is at equilibrium
Worked Example
A 1.70 x 10-3 M solution of calcium nitrate is mixed with an equal volume of 1.50 x 10-3 potassium sulfate.
Predict whether calcium sulfate (Ksp = 2.00 x 10-5) will precipitate.
Answer:
Step 1: Write the equation:
Ca(NO3)2 + K2SO4 → CaSO4 + 2KNO3
Step 2: Determine the concentration of the calcium and sulfate ions
You must half the concentrations of the solutions as you are doubling the volume
The question says you are adding equal volumes of both solutions
[Ca2+ (aq)] =
= 8.50 x 10-4 M
[SO42- (aq)] == 7.50 x 10-4 M
Step 3: Calculate Q
[Ca2+ (aq)] x [SO42- (aq)] = 8.50 x 10-4 x 7.50 x 10-4 = 6.38 x 10-7
Step 4: Compare to Ksp
Therefore, the will be no precipitation as Q is less than Ksp (of calcium sulfate)

Unlock more, it's free!
Did this page help you?