Common-Ion Effect (College Board AP® Chemistry): Study Guide
Common-Ion Effect
A saturated solution is a solution that contains the maximum amount of dissolved salt
If a second compound, which has an ion in common with the dissolved salt, is added to the saturated solution, the solubility of the salt reduces and a solid precipitate will be formed
This is also known as the common ion effect
For example, if a solution of potassium chloride (KCl) is added to a saturated solution of silver chloride (AgCl) a precipitate of silver chloride will be formed
The chloride ion is the common ion
The solubility product can be used to predict whether a precipitate will form or not
A precipitate will form if the product of the ion concentrations is greater than the solubility product (Ksp)
Common ion effect in silver chloride
When a KCl solution is added to a saturated solution of AgCl, an AgCl precipitate forms
In a saturated AgCl solution, the silver chloride is in equilibrium with its ions
AgCl (s) ⇌ Ag+ (aq) + Cl- (aq)
When a solution of potassium chloride is added:
Both KCl and AgCl have the common Cl- ion
There is an increased Cl- concentration so the equilibrium position shifts to the left
The increase in Cl- concentration also means that [Ag+ (aq)] [Cl-(aq)] is greater than the Ksp for AgCl
As a result, the AgCl is precipitated
The common ion effect with KCl (aq) and AgCl (aq)
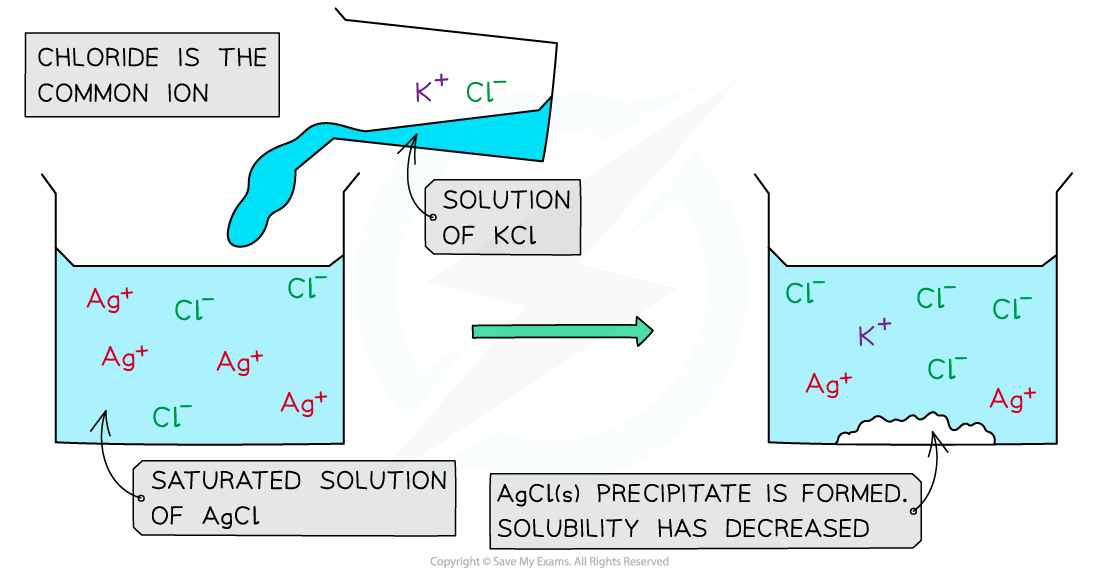
The addition of potassium chloride to a saturated solution of silver chloride results in the precipitate of silver chloride
Worked Example
Calculations using the Ksp values and the concentration of the common ion
Predict whether a precipitate of CaSO4 will form if a saturated solution of 1.0 x 10-3 M CaSO4 is mixed with an equal volume of 1.0 x 10-3 M Na2SO4.
Ksp CaSO4 = 2.0 x 10-5
Answer:
Step 1: Determine the equilibrium reaction of CaSO4:
CaSO4 (s) ⇌ Ca2+ (aq) + SO42- (aq)
Step 2: Write down the equilibrium expression for Ksp:
Ksp = [Ca2+ (aq)] [SO42- (aq)]
CaSO4 (s) | Ca2+ (aq) | + | SO42- (aq) | |
|---|---|---|---|---|
x |
| 0 |
| 0 |
-x |
| + x |
| + x |
|
| 5.0 x 10-4 |
| x + 1 x 10-3 |
Step 3: Determine the concentrations of the ions:
There are equal volumes of each solution
This means that the total solution was diluted by a factor of 2
The new concentration of the Ca2+ ion is halved:
[Ca2+] =
[Ca2+] = 5.0 x 10-4 M
The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions
Step 4: Substitute the values into the expression:
Product of the ion concentrations = [Ca2+ (aq)] x [SO42- (aq)]
Product of the ion concentrations = (5.0 x 10-4) x (1.0 x 10-3)
Product of the ion concentrations = 5.0 x 10-7 M
Step 5: Determine if a precipitate will form:
As Q (5.0 x 10-7 M) is smaller than the Ksp value (2.0 x 10-5 M), the CaSO4 precipitate will not be formed
Worked Example
The Ksp for lead chloride, PCl2 is 1.7 x 10-5 at 25 °C.
Calculate the molar solubility of PCl2 at 25 °C which is in a solution of 0.10 NaCl.
Answer:
Step 1: Write equation and expression
PbCl2 (s)
Pb2+ (aq) + 2Cl– (aq)
Ksp = [Pb2+ (aq)][Cl– (aq)]2
Step 2: Calculate concentrations of ions
PbCl2 (s) | Pb2+ (aq) | + | 2Cl– (aq) | |
|---|---|---|---|---|
x |
| 0 |
| 0 |
-x |
| + x |
| + 2x |
|
| 5.0 x 10-4 |
| 2x + 0.10 |
Step 3: Place values into expression
Ksp = [Pb2+ (aq)][Cl– (aq)]2
1.7 x 10-5 = (x)(2x + 0.10)2
The value for x is very small, so we can approximate and use 0.10 M for 2x + 0.10
1.7 x 10-5 = (x)(0.10)2
x = 1.7 x 10-3 M
Additional Note
We can compare this value of to molar solubility of PbCl2 when it is NOT in a solution of 0.10 M NaCl
Ksp = [Pb2+ (aq)][Cl– (aq)]2
1.7 x 10-5 = (x)(2x)2
1.7 x 10-5 = 4x3
x =
x = 0.016 M
So we can see that the addition of a common ion (Cl–) decreased the solubility of PbCl2 by roughly a factor of 10

Unlock more, it's free!
Did this page help you?