Representations of Equilibrium (College Board AP® Chemistry): Study Guide
Representations of Equilibrium
Particulate diagrams can be used to represent a reversible reaction over time
When no observable changes occur to the number of particles in a system involving a reversible reaction, the reaction has reached a state of equilibrium
Consider the following reaction over a period of 80 seconds
N2O4 (g) ⇌ 2NO2 (g)
Particulate representation for a reversible reaction
Particulate representation for the reversible reaction N2O4 (g) ⇌ 2NO2 (g) showing that equilibrium is established when the number of particles of each species remains constant over time
A particulate diagram of a reversible reaction at a certain point in time can be used to calculate K
For the generic equation:
aA + bB ⇌ cC + dD
A particulate representation can also be used to calculate Q and determine if the reversible reaction has reached equilibrium
The equations for Qc and QP are identical to those for K, but Q can be determined using concentration or partial pressure values from any point during the reaction
At equilibrium, Q = K
Worked Example
A2 (g) + 2B (g) ⇌ A2B2 (g)
Gases A2 and B are placed in an empty 1.00 L rigid vessel at a constant temperature of 25°C. The particle diagram below represents the contents of the vessel after equilibrium is established. Assume that each particle in the diagram represents 1 mole. Based on this information, what is the equilibrium constant, Kc, for the reaction above at 25°C?
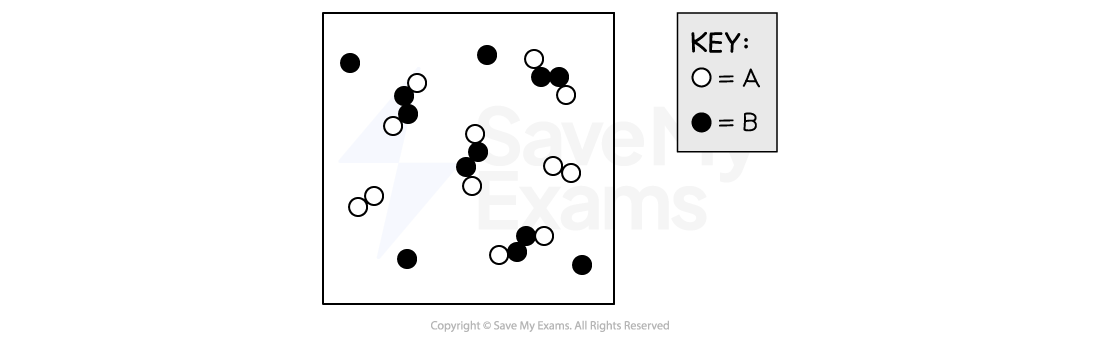
Answer:
For the generic equation
aA + bB ⇌ cC + dD
The equilibrium expression for Kc is
So the equilibrium expression for Kc for the given reaction is
Each particle in the diagram represents 1.00 mole and the total volume of the container is 1.00 L
Therefore, the number of particles of a species in the diagram also represents the concentration of that species at equilibrium in mol/L
The equilibrium concentrations are
[A2B2]eq = 4.00 mol/L
[A2]eq = 2.00 mol/L
[B]eq = 4.00 mol/L
Substituting the concentrations into the equilibrium expression
Worked Example
2X (g) ⇌ Y (g)
At 25°C, the equilibrium constant, Kc, for the reaction shown above is 15. Which of the following particle diagrams best represents the system at equilibrium? Assume that each particle in the diagrams represents a concentration of 0.1 M.
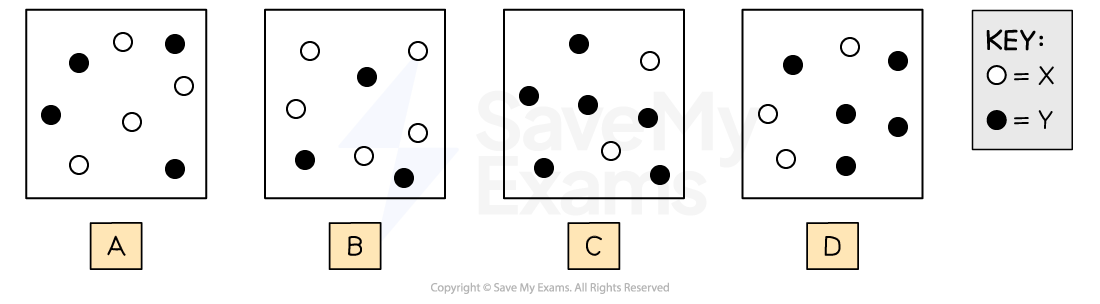
Answer:
Step 1: Determine the expression for Q
For the generic equation
aA + bB ⇌ cC + dD
The equilibrium expression for Qc is
So the expression for Qc for the given reaction is
Step 2: Solve for Q
Diagram A
[Y] = 0.4 M
[X] = 0.4 M
Diagram B
[Y] = 0.3 M
[X] = 0.5 M
Diagram C
[Y] = 0.6 M
[X] = 0.2 M
Diagram D
[Y] = 0.5 M
[X] = 0.3 M
A system is in equilibrium when Q = K
So diagram C best represents the system at equilibrium
Examiner Tips and Tricks
Be sure to read a particulate diagram question carefully. An individual particle may represent one or more moles of the substance or a specific concentration. In addition, the size of the vessel, while often 1 L, may vary.

Unlock more, it's free!
Was this revision note helpful?
