Calculating Equilibrium Concentrations (College Board AP® Chemistry): Study Guide
Calculating Equilibrium Concentrations
The concentration or partial pressure of each species at equilibrium can be determined if all of the following are known:
The balanced chemical equation
The initial concentration or partial pressure of each species
The equilibrium constant (K)
To determine the conditions at equilibrium, we use an ICE table
| Reactant | Product |
|---|---|---|
Initial |
|
|
Change |
|
|
Equilibrium |
|
|
An ICE table can be used to solve for concentrations (M), amounts (mol), or partial pressures (atm)
The initial concentration or partial pressure of each species is typically provided in the question
The change may be indicated in the question, but can also be determined by the stoichiometric coefficients
Species that are consumed during the course of the reaction will have a change of -x multiplied by the appropriate stoichiometric coefficient
Species that are produced during the course of the reaction will have a change of +x multiplied by the appropriate stoichiometric coefficient
The equilibrium concentration or partial pressure of each species is the sum of the initial and change values
We then solve for x by substituting the equilibrium values into the appropriate equilibrium expression
Once x is known, we can return to the ICE table to calculate the equilibrium concentrations or partial pressures
Worked Example
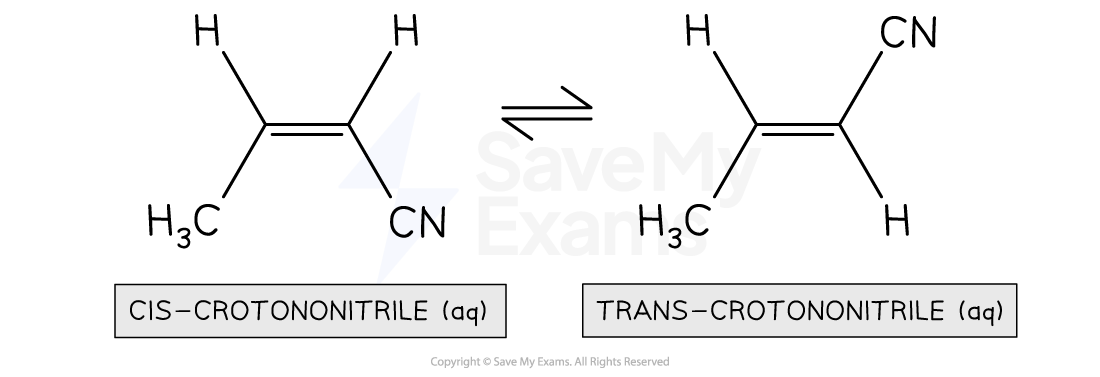
A 2.0 M sample of cis-crotononitrile at 303°C is allowed to come to equilibrium according to the equation above. The equilibrium constant, Kc, for this reaction at 303°C is 1.40. What are the concentrations of cis-crotononitrile and trans-crotononitrile after equilibrium is established?
Answer:
Step 1: Completing an ICE table
The initial concentrations of the cis- and trans- forms are 2.0 M and 0.0 M respectively
In order to reach equilibrium, there must be an overall net conversion of reactants to products as no trans-crotononitrile is initially present
Both stoichiometric coefficients are 1.
We complete the ICE table as shown below
| cis-crotononitrile | trans-crotononitrile |
|---|---|---|
Initial concentration (M) | 2.00 | 0 |
Change in concentration (M) | -x | +x |
Equilibrium concentration (M) | 2.00 - x | x |
Step 2: Using an equilibrium expression to solve for x
The equilibrium expression for Kc is
Substituting the equilibrium constant and equilibrium concentrations into the equation and rearranging to solve for x
1.17 M = x
Step 3: Calculating the concentrations at equilibrium
[cis-crotononitrile]eq = 2.00 M – x
= 2.00 M - 1.17 M
= 0.83 M
[trans-crotononitrile] = x
= 1.17 M
Worked Example
I2 (g) + H2 (g) ⇌ 2HI (g) KP = 160 at 500 K
Equimolar amounts of I2 (g) and H2 (g) were pumped into a previously evacuated, rigid sealed flask. The gases were allowed to react at 500 K according to the equation above. What is the partial pressure of HI (g) at equilibrium if the initial pressure inside the flask was 12.00 atm?
Answer:
Step 1: Completing an ICE table
Initial partial pressure
as no products are initially present
Equimolar amounts of I2 and H2 are initially present, so they must have equivalent mole fractions and therefore equal partial pressures
as the total initial pressure is 12.00 atm
In order to reach equilibrium, there must be an overall net conversion of reactants to products as no HI is initially present
The change in equilibrium must reflect the stoichiometric coefficients
We complete the ICE table as shown below
| I2 | H2 | HI |
|---|---|---|---|
Initial partial pressure (atm) | 6.00 | 6.00 | 0 |
Change in partial pressure (atm) | -x | -x | +2x |
Equilibrium partial pressure (atm) | 6.00 - x | 6.00 - x | 2x |
Step 2: Using an equilibrium expression to solve for x
The equilibrium expression for KP is
Substituting the equilibrium constant and partial pressures at equilibrium into the equation and rearranging to solve for x
Note that the right side of the equation is a perfect square
Step 3: Calculating the partial pressure of HI at equilibrium
From the ICE table
Substituting x into the equation
Examiner Tips and Tricks
Check your calculations by substituting your equilibrium concentrations or partial pressures back into the equilibrium expression. The value you determine for K should be close to the original value given in the question.
Always check the equilibrium expression for perfect squares. Taking the square root of both sides of the equation in these instances allows you to quickly simplify the calculation.

You've read 0 of your 5 free study guides this week
Unlock more, it's free!
Did this page help you?