Introduction to Rate Law (College Board AP® Chemistry): Study Guide
Rate Law
Experimental data and the rate of reaction
One way to study the effect of concentration on the rate is monitoring the rate of reaction against the concentration of the reactant
The following general reaction will be used as example
D → E + F
The rate of reaction at different concentrations of D was tabulated and plotted
[D] (M) | Rate (M s-1) |
|---|---|
3.00 | 2.000 x 10-3 |
2.00 | 1.334 x 10-3 |
1.00 | 6.670 x 10-4 |
A line is plotted and the gradient of the line is calculated using the formula below
Graph to show rate against [D]
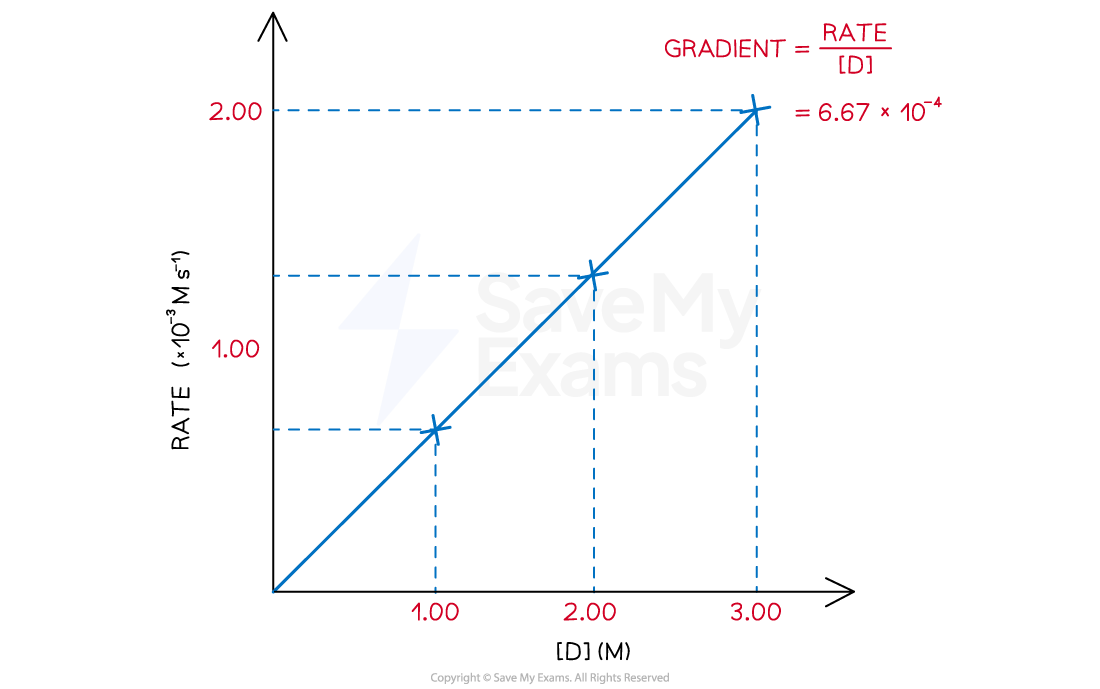
Rate of reaction over various concentrations of D
As shown in the diagram, there is a directly proportional relation between the rate of reaction and the concentration of D
This relation can be written as a mathematical equation as shown below
Rate ∝ [D]
Rate = k [D]
This equation means that if the concentration of D doubles, the rate will double
The constant of proportionality (k) is called the rate constant
The units of the rate constant can be used to determine the order of the reaction
The rate equation
The following general reaction will be used to discuss the rate equation:
A +B → C+D
The general rate equation is:
Rate = k [A]m [B]n
Where:
[A] and [B] are the concentration of reactants
m and n are the orders with respect to each reactant
The rate of the reaction will depend on the mechanism of reaction and it can only be found experimentally
Intermediate products do not feature in rate equations
The order of reactants
The order of reactants shows how the concentration of a reactant affect the rate
It is represented as the power to which the concentration is raised in the rate equation
The most common orders are listed below:
Zero order occurs when the order respect to a chemical is 0
This means that changing the concentration has no effect on the rate
Therefore, it is not included in the rate equation
First order occurs when the order respect to a chemical is 1
This means that the concentration is directly proportional to the rate
E.g. Doubling the concentration will double the rate
Second order occurs when the order respect to a chemical is 2
This means the that square of the concentration is directly proportional to the rate
E.g. Doubling the concentration will increase the rate by a factor of 4 since 22 is 4
Overall order is just the sum of the powers of the reactants that appear in the rate equation
Worked Example
The chemical equation for the thermal decomposition of dinitrogen pentoxide is:
2N2O5 (g) → 4NO2 (g) + O2 (g)
The rate equation for this reaction is:
Rate = k [N2O5 (g)]
State the order of reaction with respect to nitrogen pentoxide
State the overall order of reaction
Deduce the effect on the rate of reaction if the concentration of dinitrogen pentoxide is doubled
Determine the units of the rate constant
Answers:
Answer 1:
Dinitrogen pentoxide features in the rate equation, therefore, it cannot be order zero
The dinitrogen pentoxide is not raised to a power, which means that it cannot be order 2
Therefore, the order with respect to dinitrogen pentoxide must be order 1
Answer 2:
The overall order is just the sum of all the powers in the rate equation. Since, dinitrogen pentoxide is the only reactant and it is raised to the power of 1. The overall order is first order
Answer 3:
Since the overall order is first order, the concentration of dinitrogen pentoxide is directly proportional to the rate
This means that if the concentration of the dinitrogen pentoxide is doubled, then the rate of reaction will be the double
Answer 4:
Rearranging the equation,
Examiner Tips and Tricks
The overall order of the reaction can be inferred from the units of the rate constant. If the rate is measure per second:
Rate constant units of a zero order reaction are M s-1
Rate constant units of a first order reaction are s-1
Rate constant units of a second order reaction are M-1 s-1
Initial Rates & Orders of Reaction
The order of the reactants can be determined with experimental data from the the initial rates of reaction
This information is usually displayed in a table
The following steps must be followed to determine the order for each reactant:
Identify two experiments where the concentration of this reactant changes, but the concentration of the other reactants is the same
Identify what has happened with the concentration
Determine the effect of the concentration in the rate of reaction
Deduce the order of reaction
Repeat this procedure with all the reactants one at the time
Once all the orders are determined the rate equation can be built
Zero order reactants are not included
First order reactants are included and do not require a power (it is the s
Second order reactants are raised to the power of 2
Examiner Tips and Tricks
Determining the orders for each reactant is one of the most assessed tasks regarding kinetics, so it's worth spending time to get to know this topic well
Worked Example
(CH3)3CBr + OH- → (CH3)3COH + Br-
The table show experimental data for the reaction above
Experiment | Initial [(CH3)3CBr] / M | Initial [OH-] / M | Initial rate of reaction / M s-1 |
|---|---|---|---|
1 | 1.0 x 10-3 | 2.0 x 10-3 | 3.0 x 10-3 |
2 | 2.0 x 10-3 | 2.0 x 10-3 | 6.0 x 10-3 |
3 | 1.0 x 10-3 | 4.0 x 10-3 | 1.2 x 10-2 |
4 | 1.5 x 10-3 | 4.0 x 10-3 | 4.5 x 10-3 |
Determine the rate equation for this reaction
Answer:
Order with respect to (CH3)3CBr
In experiments 1 and 2, the concentration of (CH3)3CBr changes while the concentration of OH- remains constant
The [(CH3)3CBr] has doubled
The rate of the reaction has also doubled
Therefore, the order with respect to [(CH3)3CBr] is 1 (first order)
order = 1
Order with respect to OH-
In experiments 1 and 3, the concentration of OH- changes while the concentration of (CH3)3CBr remains constant
The [OH-] has doubled
The rate of the reaction has increased by a factor of 4
Therefore, the order with respect to [OH-] is 2 (second order)
[2]2 = 4
order = 2
Building the rate equation
Rate = k [(CH3)3CBr]m [OH-]n
Replacing the orders,
Rate = k [(CH3)3CBr] [OH-]2

Unlock more, it's free!
Did this page help you?