Transitions Associated with Radiation (College Board AP® Chemistry): Study Guide
Transitions Associated with Microwave Radiation
Molecules and atoms can absorb light particles (photons)
When they absorb light, the molecule or atom is promoted to an excited state
Infrared photons have low energy
When photons of microwave radiation are absorbed the rotational behavior of a molecule is stimulated
Therefore, the molecule is promoted to an excited rotational state
The different rotational axis of a molecule can be analyzed by thinking atoms as points in a 3-dimensional system of coordinates
A rotation will change the orientation of the molecule
The orientation will depend on the frequency of the photon with microwave radiation that is absorbed
Rotational axis in a water molecule

Different rotational axis in a water molecule
One of the most used examples regarding excited rotational states is the use of the microwave oven
The microwave oven transfer rotational energy to the water molecules in your food
Transitions Associated with Infrared Radiation
Molecules and atoms can absorb low energy infrared photons
When they absorb a photon, they are promoted to an excited state
When photons of infrared radiation are absorbed, the vibrational behavior of a molecule is stimulated
Therefore, the molecule is promoted to an excited vibrational state
The modes of vibration of molecules can be analyzed by understanding the covalent bonds as springs
In the same ways as springs, there are multiple ways in which a molecule can vibrate
This will depend on the frequency of the photon with infrared radiation that is absorbed
Modes of vibration for the CO2 molecule

Different modes of vibration in a CO2 molecule. Each mode has a characteristic frequency of vibration
The analytical technique that exploits the changes in vibrations of atoms to identify compounds is called infrared spectroscopy
Transitions Associated with Ultraviolet/Visible Radiation
Molecules and atoms can absorb photons in the visible and the ultraviolet region of the spectrum
When they absorb a photon, they are promoted to an excited state
The energy of visible and ultraviolet photons is high enough to stimulate electronic transitions
Therefore, the electrons are promoted from low energy orbitals to high energy orbitals
When electrons return to the ground state, energy is emitted
Absorption and Emission diagram
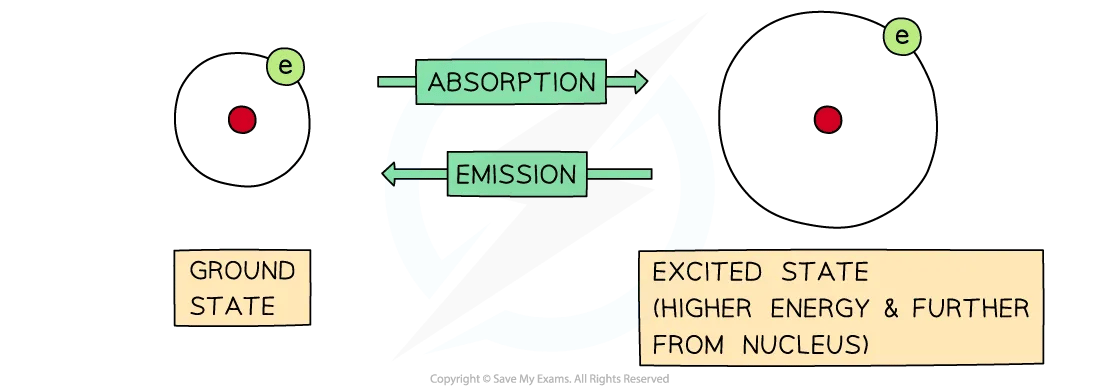
The difference between absorption and emission depends on whether electrons are jumping from lower to higher energy levels or the other way around
When an electronic transition occurs, it is usually followed by changes in the rotational and vibrational states in a molecule
The image below shows a summary that connects the specific regions of the electromagnetic spectrum and the types of molecular and electronic transitions associated with them
Photons with higher energy are needed to reach electronic excited states.
On the other hand, lower energy photons (infrared and microwave) can be absorbed to reach vibrational and rotational excited states
Summary of the molecular and electronic transitions

Electronic, vibration and rotational excited states associated with an specific region of the electromagnetic spectrum

Unlock more, it's free!
Did this page help you?