Properties of Photons (College Board AP® Chemistry): Study Guide
Photons
One of the main characteristics of light is its wave-particle duality
This means that light can behave as a wave, but also as a particle
As seen in previous sections, a light wave has frequency, wavelength and energy
However, the discovery of the photoelectric effect suggested that light can also behaves as a particle
The photoelectric effect was observed when some metals emit electrons when light was shone on them
The results of this experiment suggested that light must come into particles
A light particle is called a photon
Absorption and Emission of photons
Electrons move around the nucleus of the atoms in energy shells
Atoms and molecules can absorb photons, and the electrons will jump to higher energy shells
When this occurs, the energy of the atom or molecule increases by an amount equal to the energy of the photon
A moment later, a photon will be emitted, and the electrons will return to their original shell which is also called the ground state
When this occurs, the energy of the atom or molecule will decrease by an amount equal to the energy of the photon
Absorption and Emission diagram
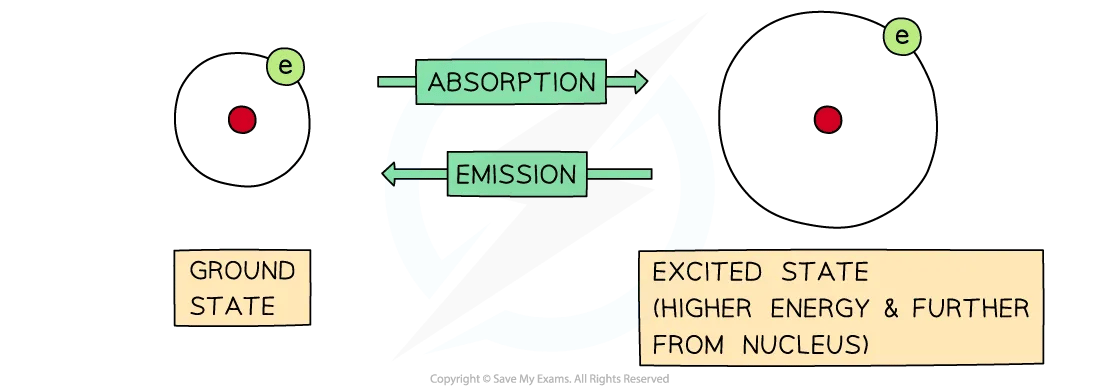
The difference between absorption and emission depends on whether electrons are jumping from lower to higher energy levels or the other way around
The Hydrogen Emission Spectrum
Niels Bohr constructed the hydrogen emission spectrum where he showed the energy released when electrons jump from higher shells to lower shells
Bohr suggested that electrons can only exist in fixed energy shells
This means that electronic transitions can only occur when photons with an specific amount of energy are absorbed or released
Each electronic transition has to do with photons from specific regions of the electromagnetic spectrum
Regions of the electromagnetic spectrum
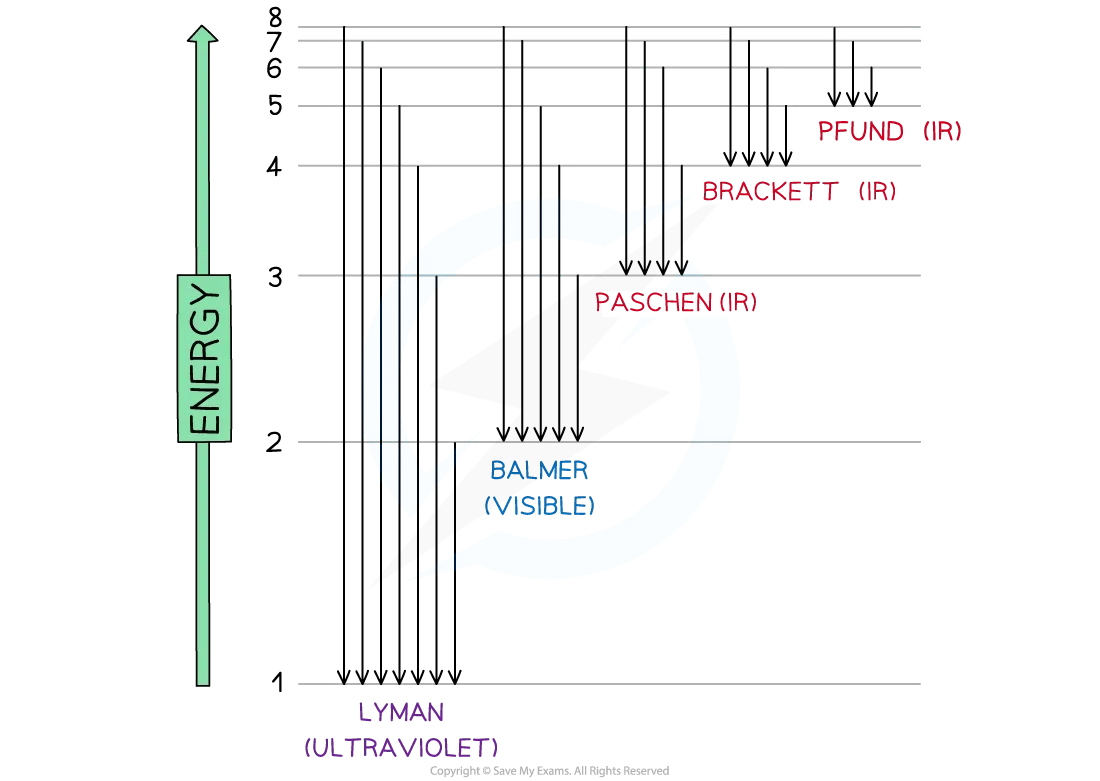
Electron jumps in the hydrogen spectrum
The jumps can be summarized in table below:
Electron transitions summary
Jumps | Region | Energy |
|---|---|---|
n∞→ n3 | Infrared | Low |
n∞ → n2 | Visible | ↓ |
n∞ → n1 | Ultraviolet | High |
Energy of Photons
Frequency, Wavelength and Energy
As it was shown in the electromagnetic spectrum, the shorter the frequency of an electromagnetic wave, the longer its wavelength
Frequency (ν) and wavelength (λ) are inversely proportional
The equation that links them is:
In this equation c is the speed of light which is 2.998 x 108 ms-1
The spectrum also shows that, the shorter the frequency, the lower the energy of the radiation
Frequency (ν) and energy (E) are directly proportional
The equation that links the energy emitted or absorbed by a photon and its frequency is:
In this equation h is the Planck’s constant, which is 6.626 x 10-34 J s
These two equations can be used to calculate frequency, wavelength or energy when one of them is given
Examiner Tips and Tricks
Both equations and constants can be found in the AP Chemistry Constants and Equations section in your examination
Worked Example
Calculate the wavelength of the photon emitted when an electron transition has released 1.632 x 10 -18 J
Answer:
Step 1: Calculate the frequency of the photon by using the Planck’s equation
v = 2.463 × 1015 s-1
Step 2: Use the frequency calculated in Step 1 to calculate the wavelength of the photon
λ = 1.217 × 10-7 m

Unlock more, it's free!
Did this page help you?