Mass Spectrum of an Element (College Board AP® Chemistry): Study Guide
Mass Spectrum of an Element
The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer
A mass spectrometer provides this information in the form of a mass spectrum
Sample mass spectrum
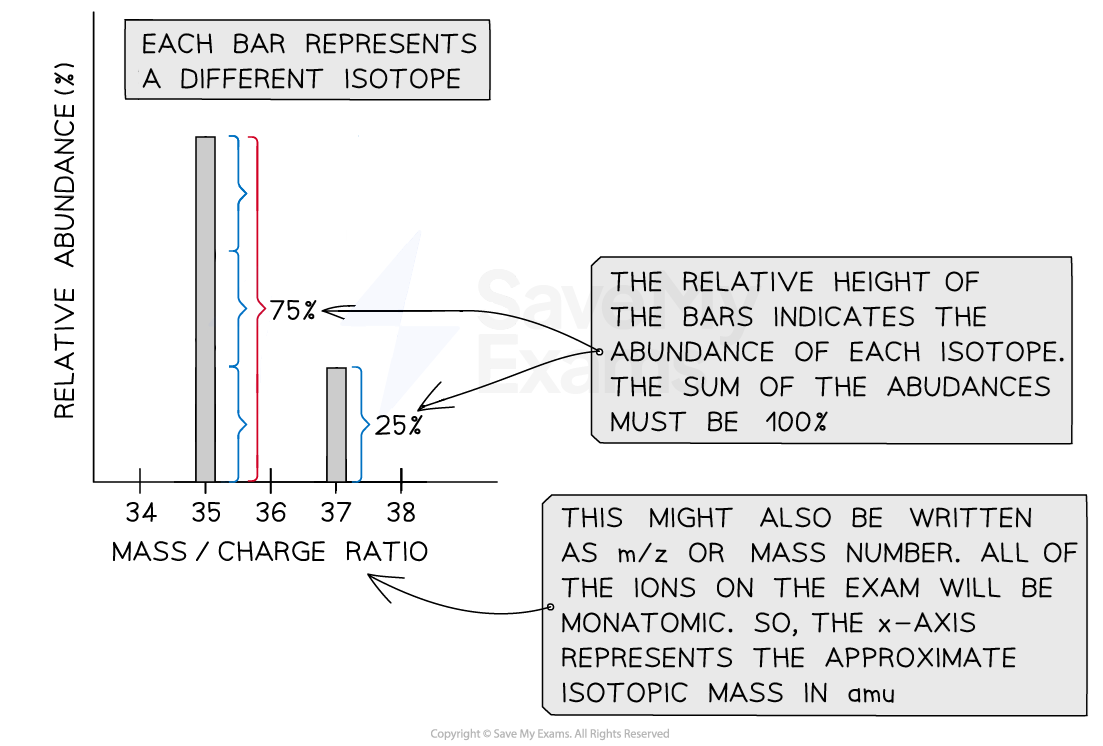
The mass spectrum of chlorine with peaks for the isotopes chlorine-35 and chlorine-37 have abundances of 25% and 75% respectively
The average atomic mass can be estimated and the identity of an element determined using the approximate isotopic mass and abundance from a mass spectrum
Worked Example

Based on the mass spectrum of a pure element shown above, what is the most likely identity of the element?
Cu
Zn
Ga
Ni
Answer:
The average atomic mass of an element must fall between the masses of its lightest and heaviest isotopes
The element cannot be Ni (58.69 amu)
The average atomic mass will most likely be close in value to the mass of the isotope with the highest abundance
The element cannot be Ga (69.72 amu)
The average atomic mass of the element will be near 64 amu, but the element also has isotopes of significant abundance with masses of 66 and 68
This will skew the average atomic mass to be greater than 64 amu
So, the element is Zn (65.38 amu), not Cu (63.55 amu)
Examiner Tips and Tricks
Note that some textbooks will use the terms relative abundance and percent abundance interchangeably.

Unlock more, it's free!
Did this page help you?