Proteins (College Board AP® Biology): Study Guide
Structure & function in proteins
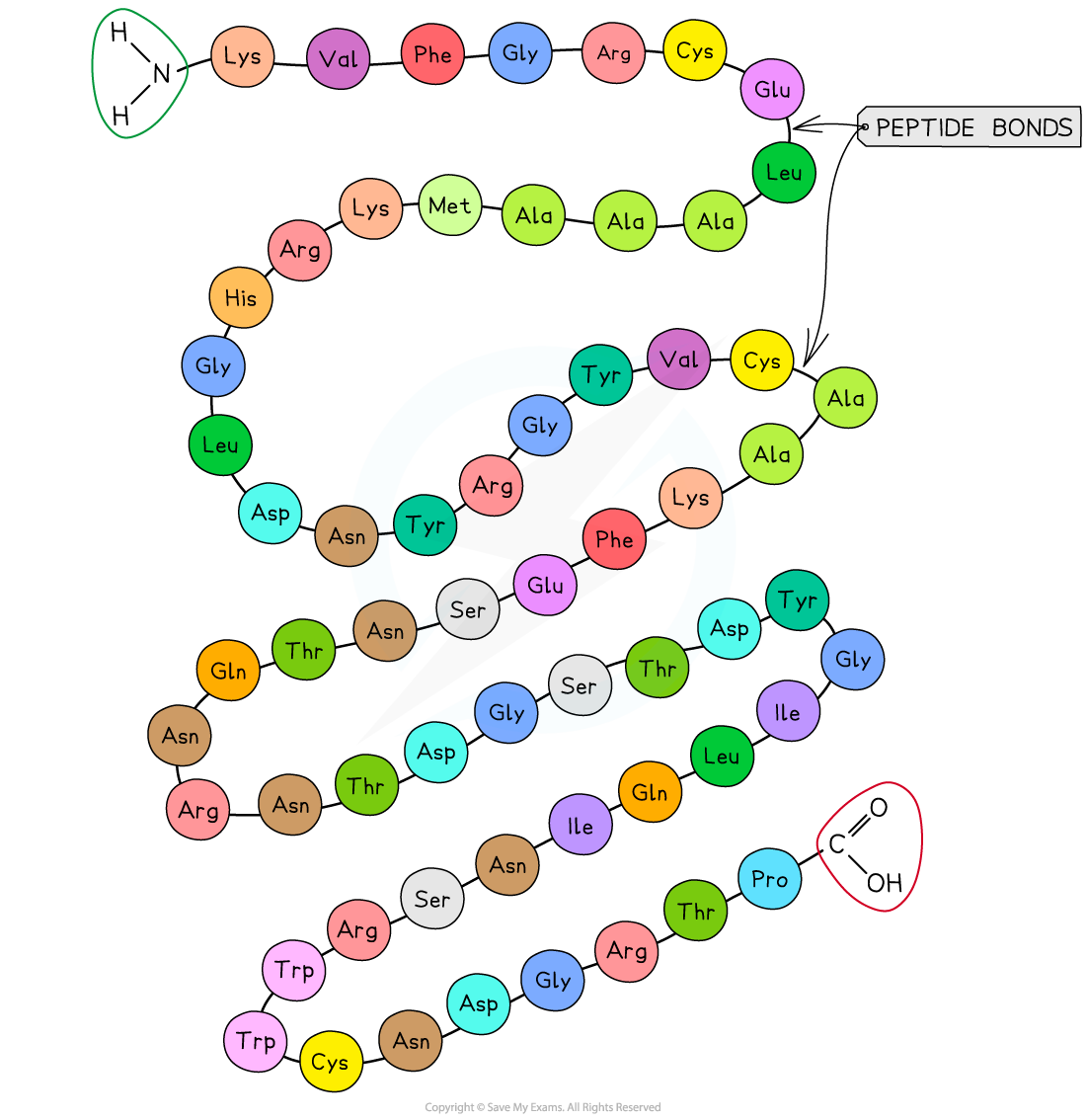
Proteins are made of linear chains of monomers called amino acids
The amino acids are connected by covalent bonds (peptide bonds) at one end of the growing peptide chain
The sequence, type and number of the amino acids within a protein determine its shape and, therefore, its function
Proteins are extremely important in cells because they form all of the following:
enzymes
cell membrane proteins, e.g. carriers
hormones
immunoproteins, e.g. immunoglobulins
transport proteins, e.g. hemoglobin
structural proteins, e.g. keratin and collagen
contractile proteins, e.g. myosin
All genes that are expressed will code for specific proteins; therefore, all of the reactions necessary for life are dependent on the function of proteins
Amino acid structure
Amino acids are the monomers of polypeptides
There are 20 amino acids found in polypeptides common to all living organisms
The specific order of amino acids in the overall protein polypeptide (referred to as its primary structure) determines the overall shape of the protein
Within an amino acid, a central carbon atom is bonded to:
an amino terminus (NH2)
a carboxylic acid terminus (COOH)
a hydrogen atom
an R group
The R group of an amino acid can be categorized by three possible chemical properties: hydrophobic/nonpolar, hydrophilic/polar, or ionic
The interactions of these R groups determine the structure and function of that region of the protein

Peptide bonds
Amino acids are connected by the formation of peptide bonds at the carboxyl terminus of the growing peptide chain.
To form a peptide bond:
a hydroxyl group (-OH) is lost from the carboxylic group (-COOH) of one amino acid
a hydrogen atom is lost from the amino group (-NH2) of the neighboring amino acid
The remaining carbon atom (with the double-bonded oxygen) from the first amino acid bonds to the nitrogen atom of the second amino acid
This is a dehydration synthesis reaction, so water is released
Dipeptides are formed by the dehydration synthesis of two amino acids
Polypeptides are formed by the dehydration synthesis of many (three or more) amino acids
During hydrolysis reactions, the addition of water breaks the peptide bonds, resulting in polypeptides being broken down into amino acids

Examiner Tips and Tricks
You will be expected to recognize whether an unfamiliar molecule is an amino acid or polypeptide, so look for the functional groups (amine and carboxyl). When asked to identify the location of the peptide bond, look for where nitrogen is bonded to a carbon that has a double bond with an oxygen atom. The molecular structure of specific amino acids is beyond the scope of the AP Exam.
Also, note that the R group is not involved in the formation of a peptide bond.
Categories of amino acid by R group
The R Groups of the 20 amino acids fall into 3 categories
These are based on the properties of the side chains (R groups)
Hydrophobic (nonpolar side chains)
Hydrophilic (polar side chains)
Some hydrophilic amino acids are acidic or basic, based on the ionization of their side chain groups (-COOH or -NH2)
These side chains are distinct from the -COOH and -NH2 groups that all amino acids possess attached to their central carbon atom
The interactions between the different R groups in a polypeptide chain determine the 3-D shape of the protein
Examiner Tips and Tricks
You are not expected to have memorized the R groups of the 20 amino acids, although you should be able to recognize from a molecular diagram whether an amino acid is hydrophobic, hydrophilic, acidic, basic etc.
Protein structure
There are four levels of structure in proteins:
Three are related to a single polypeptide chain and the fourth level relates to a protein that has two or more polypeptide chains
The four elements of protein structure determine the function of a protein
Polypeptide or protein molecules can have anywhere from three amino acids (Glutathione) to more than 34,000 amino acids (Titan) bonded together in chains
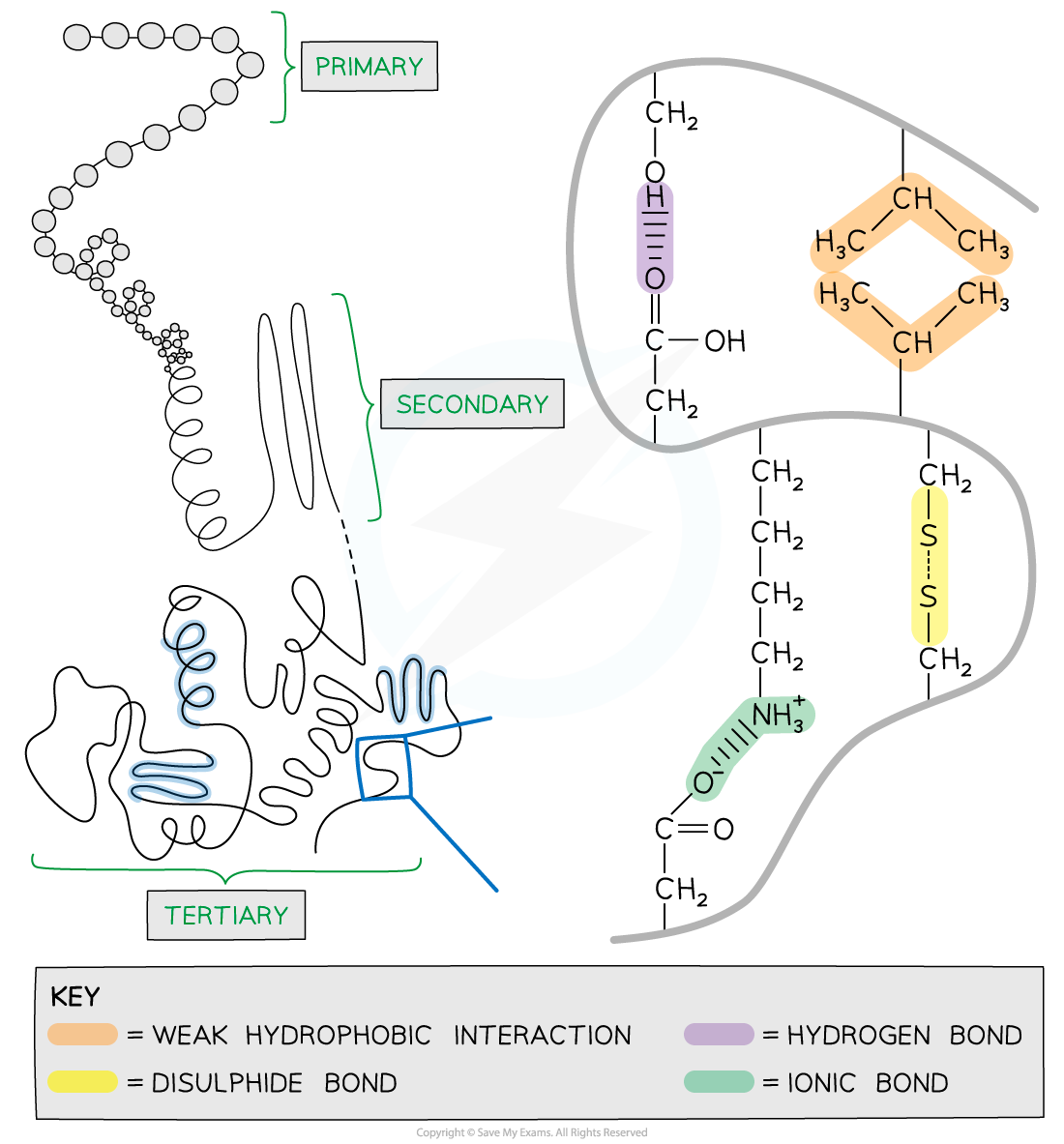
Primary
Proteins have a primary structure determined by the sequence order of their amino acids
DNA within a cell determines the primary structure of a protein by instructing the cell to add certain amino acids in specific quantities in a certain sequence
This affects the shape and, therefore the function of the protein
The primary structure is specific for each protein (one alteration in the sequence of amino acids can affect the function of the protein)
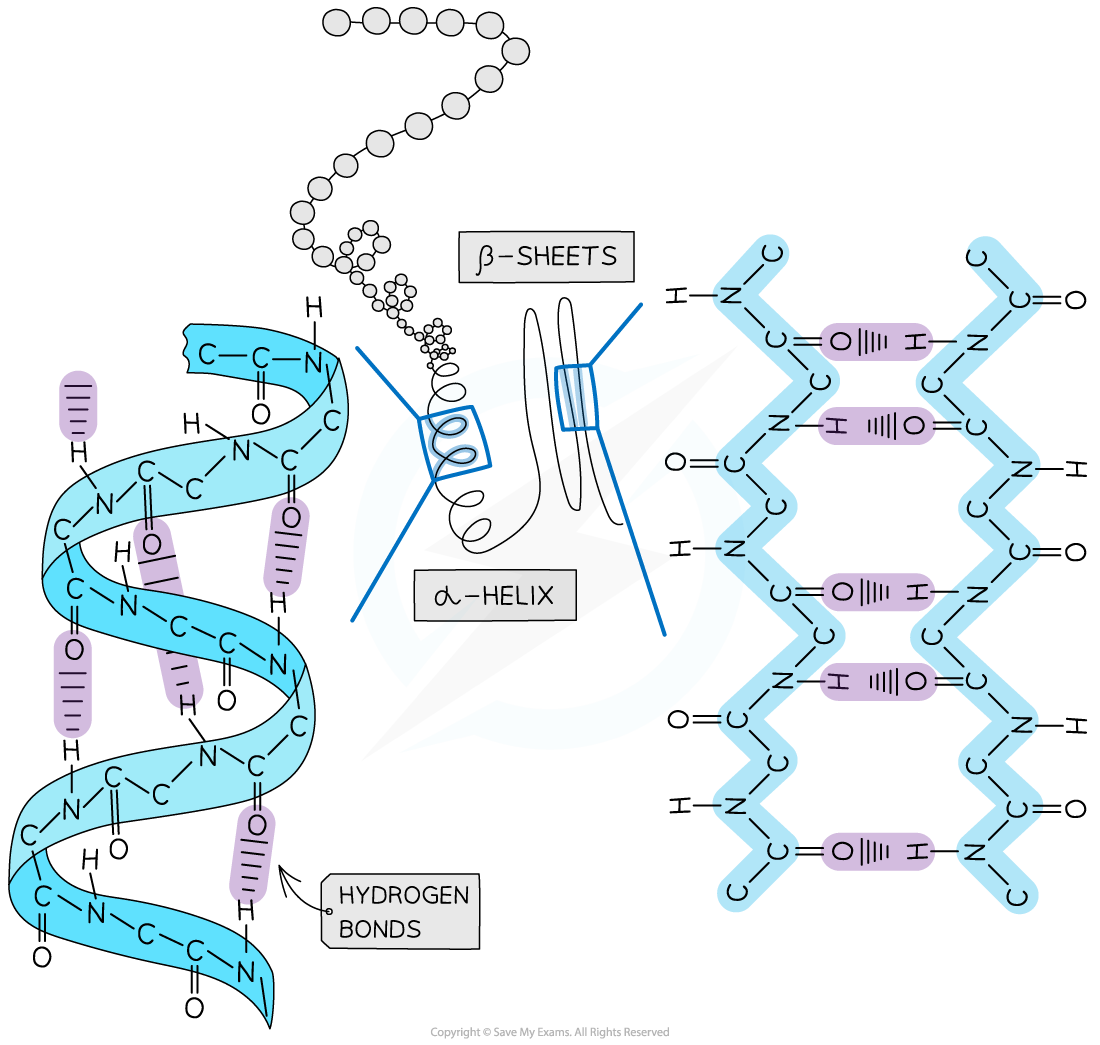
Secondary
The secondary structure arises from the local folding of the amino acid chain into different shapes
The secondary structure of a protein is held together by hydrogen bonds that form between the -NH region of one amino acid and the -C=O region of another
The hydrogen of -NH has an overall positive charge while the oxygen of -C=O has an overall negative charge
Two shapes can form within proteins due to the hydrogen bonds:
α-helix
β-pleated sheet
The α-helix shape occurs when the hydrogen bonds form between every fourth peptide bond
The β-pleated sheet shape forms when the protein folds so that two parts of the polypeptide chain are parallel to each other, enabling hydrogen bonds to form between the folded layers
Hydrogen bonds are relatively weak, so they can be broken easily by high temperatures and pH changes
Tertiary
Tertiary structure is the overall three-dimensional shape of the protein
Additional bonds are formed between the R groups of the amino acids
The additional bonds can be:
hydrogen bonds between R groups
disulphide bonds between cysteine amino acids
ionic bonds between charged R groups
weak hydrophobic interactions between non-polar R groups
Tertiary structure is common in globular proteins
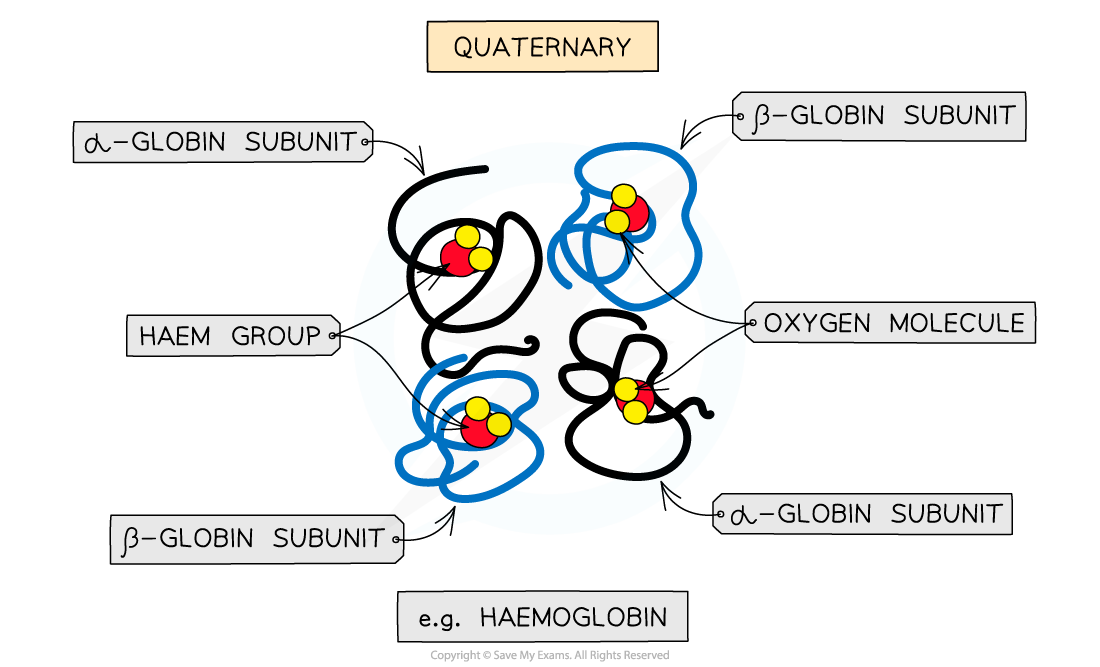
Quaternary
Quaternary structure arises from interactions between multiple polypeptide units where more than one polypeptide chain works together as a functional macromolecule, e.g. haemoglobin
Each polypeptide chain in the quaternary structure is referred to as a subunit of the protein

Unlock more, it's free!
Did this page help you?