An isotope of polonium-213 () first decays into an isotope of lead-209 (
) and this lead isotope then decays into the stable isotope of bismuth (Bi). Fig. 24 shows two arrows on a neutron number N against proton number Z chart to illustrate these two decays.
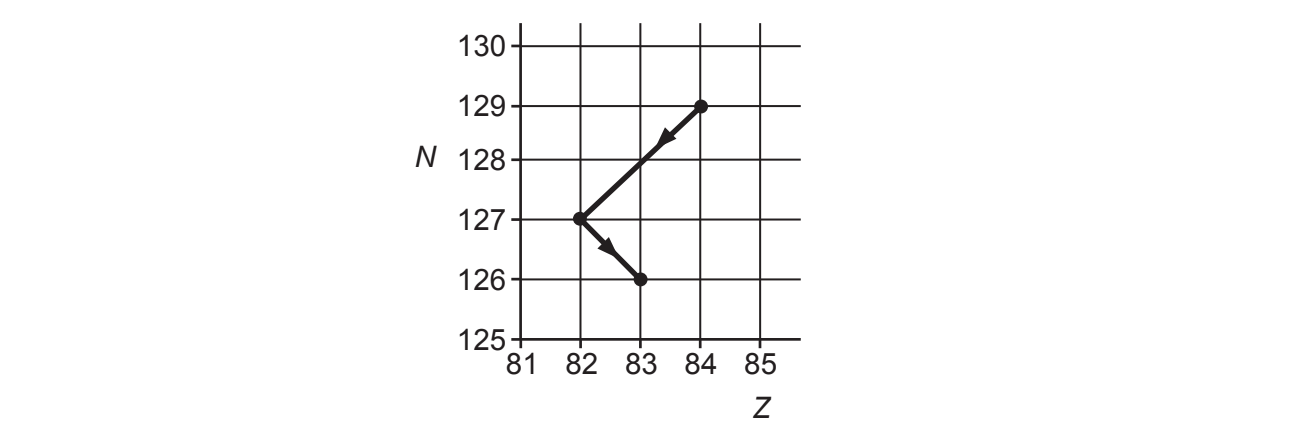
Fig. 24
Complete the nuclear decay equations for
i) the polonium isotope
[1]
ii) the lead isotope
[2]
A pure sample of polonium-213 is being produced in a research laboratory. The half-life of is very small compared with the half-life of
.
After a very short time, the ionising radiation detected from the sample is mainly from the beta-minus decay of the lead-209 nuclei.
i) Briefly describe and explain an experiment that can be carried out to confirm the beta-minus radiation emitted from the lead nuclei.
[2]
ii) The activity of the sample of after 7.0 hours is 12 kBq.
The half-life of is 3.3 hours.
Calculate the initial number of lead-209 nuclei in this sample.
number of nuclei = ......................................................... [4]
Did this page help you?


