Radioactive Decay Equations (OCR A Level Physics): Revision Note
Exam code: H556
Radioactive Decay Equations
In radioactive decay, the number of undecayed nuclei falls very rapidly, without ever reaching zero
Such a model is known as exponential decay
The graph of number of undecayed nuclei against time has a very distinctive shape:
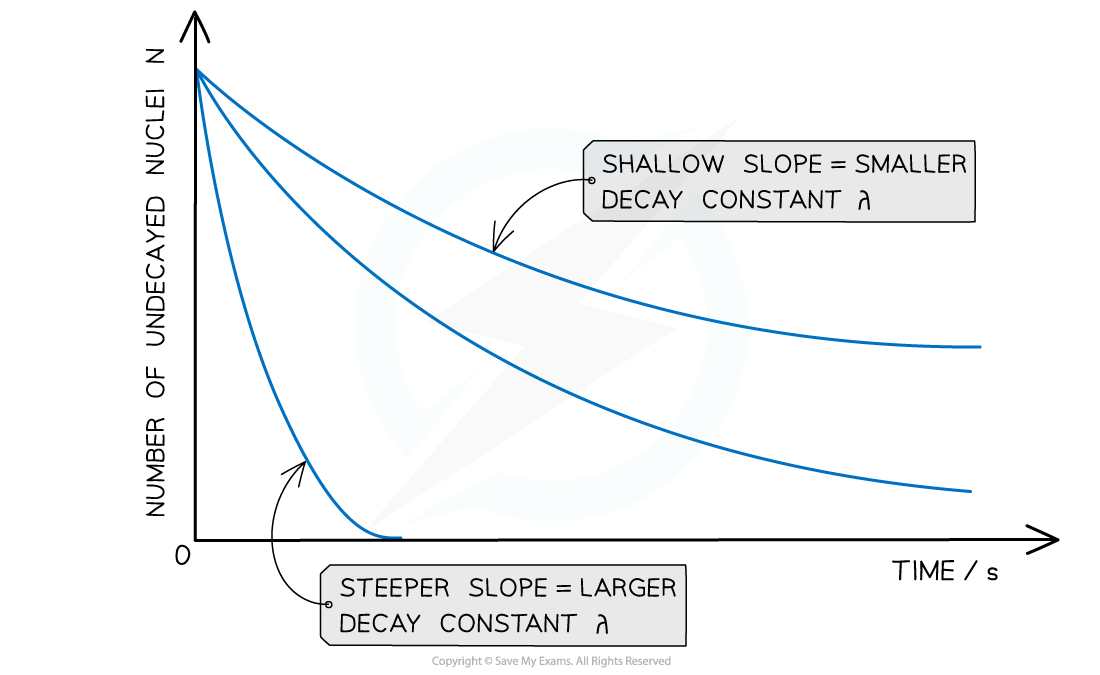
Radioactive decay follows an exponential pattern. The graph shows three different isotopes each with a different rate of decay
The key features of this graph are:
The steeper the slope, the larger the decay constant λ (and vice versa)
The decay curves always start on the y-axis at the initial number of undecayed nuclei (N0)
Equations for Radioactive Decay
The number of undecayed nuclei N can be represented in exponential form by the equation:
Where:
N0 = the initial number of undecayed nuclei (when t = 0)
N = number of undecayed nuclei at a certain time t
λ = decay constant (s-1)
t = time interval (s)
The number of nuclei can be substituted for other quantities.
For example, the activity A is directly proportional to N, so it can also be represented in exponential form by the equation:
Where:
A = activity at a certain time t (Bq)
A0 = initial activity (Bq)
The received count rate C is related to the activity of the sample, hence it can also be represented in exponential form by the equation:
Where:
C = count rate at a certain time t (counts per minute or cpm)
C0 = initial count rate (counts per minute or cpm)
The exponential function e
The symbol
represents the exponential constant
It is approximately equal to
= 2.718
On a calculator it is shown by the button
The inverse function of
is
, known as the natural logarithmic function
This is because, if
, then
Worked Example
Strontium-90 decays with the emission of a β-particle to form yttrium-90.
The decay constant of strontium-90 is 0.025 year -1.
Determine the activity of the sample after 5.0 years, expressing the answer as a fraction of the initial activity
.
Answer:
Step 1: Write out the known quantities
Decay constant, λ = 0.025 year -1
Time interval, t = 5.0 years
Both quantities have the same unit, so there is no need for conversion
Step 2: Write the equation for activity in exponential form
Step 3: Rearrange the equation for the ratio between A and A0
Step 4: Calculate the ratio A/A0
Therefore, the activity of strontium-90 decreases by a factor of 0.88, or 12%, after 5 years

Unlock more, it's free!
Did this page help you?