AZX Notation & Isotopes (OCR A Level Physics): Revision Note
Exam code: H556
AZX Notation & Isotopes
AZX Notation
A nuclide is a group of atoms containing the same number of protons and neutrons
For example, 5 atoms of oxygen are all the same nuclide but are 5 separate atoms
Atomic symbols are written in a specific notation called nuclide or AZX notation
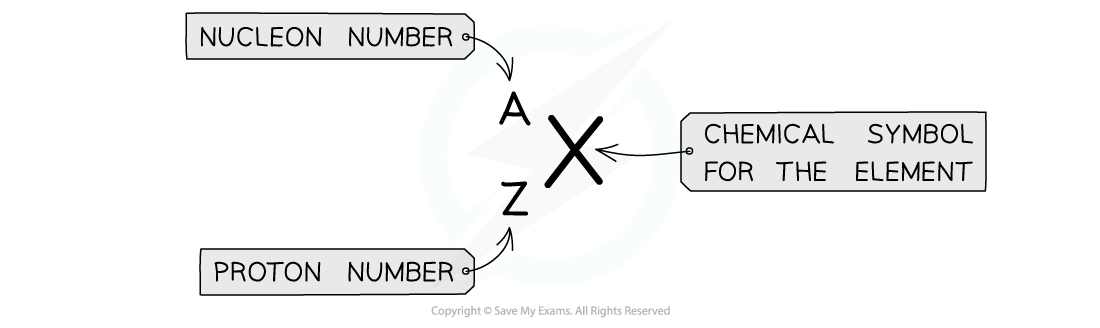
Atomic symbols in AZX Notation describe the constituents of nuclei
The top number A represents the nucleon number or the mass number
Nucleon number (A) = total number of protons and neutrons in the nucleus
The lower number Z represents the proton or atomic number
Proton number (Z) = total number of protons in the nucleus
Note: In Chemistry, the nucleon number is referred to as the mass number and the proton number as the atomic number. The periodic table is ordered by atomic number
Isotopes
Although all atoms of the same element always have the same number of protons (and hence electrons), the number of neutrons can vary
An isotope is defined as:
An atom (of the same element) that has an equal number of protons but a different number of neutrons
Hydrogen has two isotopes: deuterium and tritium
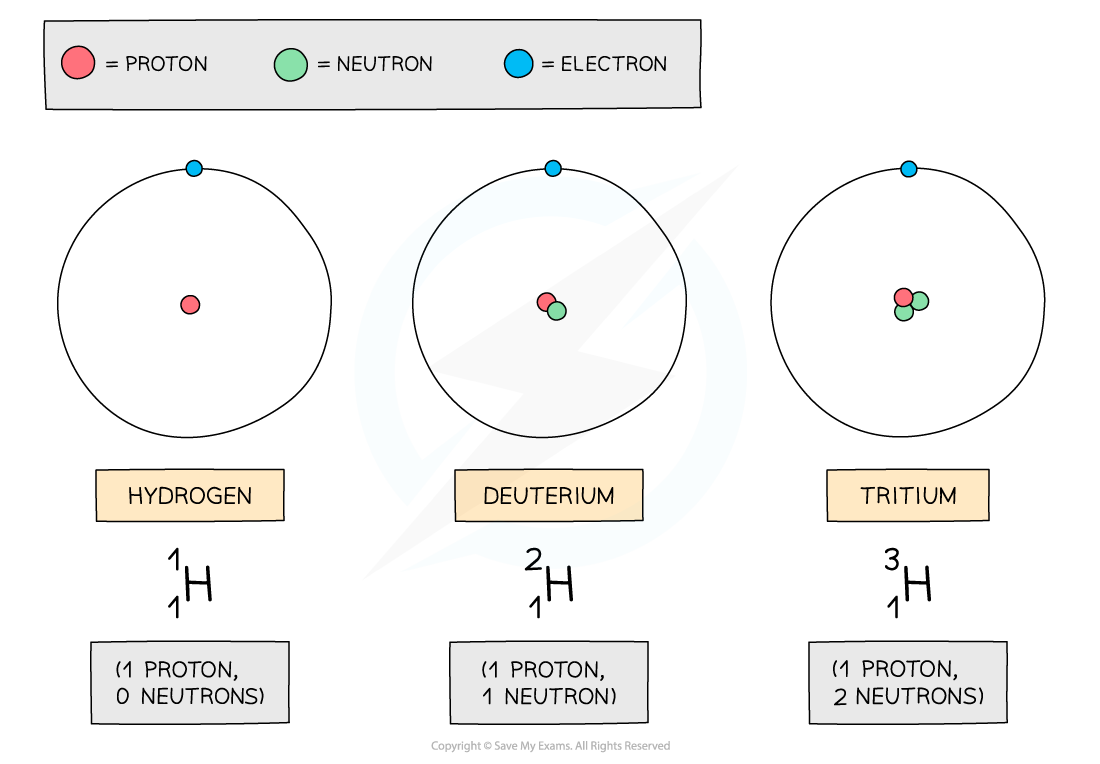
The three atoms shown above are all forms of hydrogen, but they each have different numbers of neutrons
The neutron number of an atom is found by subtracting the proton number from the nucleon number
Since nucleon number includes the number of neutrons, an isotope of an element will also have a different nucleon / mass number
Since isotopes have an imbalance of neutrons and protons, they are unstable
This means they decay and emit radiation to achieve a more stable form
This can happen from anywhere between a few nanoseconds to 100,000 years
Differences Between Isotopes
The number of neutrons in an atom does not affect the chemical properties of an atom, such as its charge, but only its mass
This is because neutrons have no charge but do have mass
The charge of the nucleus of a particular element is always the same
In the periodic table, the mass number of Chlorine is often given as 35.5

This section of a periodic table shows Chlorine as having a mass number of 35.5, but other elements have an integer mass number
The mass number of chlorine is given as 35.5 because it has 2 isotopes, one with a mass number of 35 and the other with a mass number of 37
Chlorine-35 is about three times more abundant than chlorine-37, so the given mass number of chlorine is closer to 35 than 37 because the mass number is a weighted average, therefore it takes into account the proportion of each isotope present
The number of electrons and protons in different isotopes remains the same
Some isotopes are unstable as they have an imbalance of protons and neutrons
Worked Example
One of the rows in the table shows a pair of nuclei that are isotopes of one another.

Which row is correct?
Answer: B
Step 1: State the properties of isotopes
Isotopes are nuclei with the same number of protons but different number of neutrons
The nucleon number is the sum of the protons and neutron
Therefore, an isotope has a different nucleon number too
Step 2: Calculate number of protons in the first nucleus
Nucleon number: 37
Neutrons: 20
Protons = 37 − 20 = 17
Step 3: Calculate number of protons in the second nucleus
Nucleon number: 35
Neutrons: 18
Protons = 35 − 18 = 17
Step 4: Conclusion
Therefore, they have the same number of protons but different numbers of neutrons and are isotopes of each other
The correct answer is therefore option B

Unlock more, it's free!
Did this page help you?