Atomic Structure (OCR A Level Physics) : Revision Note
Atomic Structure
The atoms of all elements are made up of three types of particles: protons, neutrons and electrons.

Protons and neutrons are found in the nucleus of an atom while electrons orbit the nucleus
The properties of each particle in SI units are shown in the table below:

It is important to know the relative masses and charges that protons, neutrons and electrons have in relation to each other
The relative properties of each particle are shown in the table below:

A stable atom is neutral (it has no charge)
Since protons and electrons have the same charge, but opposite signs, a stable atom has an equal number of both for the overall charge to remain neutral
Examiner Tips and Tricks
Remember not to mix up the ‘atom’ and the ‘nucleus’. The ‘atom’ consists of the nucleus and electrons. The ‘nucleus’ just consists of the protons and neutrons in the middle of the atom, not the electrons.
Relative Sizes of the Atom and Nucleus
Relative Sizes
From Rutherford's Alpha Scattering Experiment we know that most of an atom is made up of empty space

An atom: a small positive nucleus, surrounded by negative electrons
The atom is around 100,000 times larger than the nucleus!
The diameter of an atom is 1 x 10−10 m
The diameter of a nucleus is 1 x 10−15 m
So, 1 x 10-15 x 100,000 = 1 x 10−10
Closest Approach Method
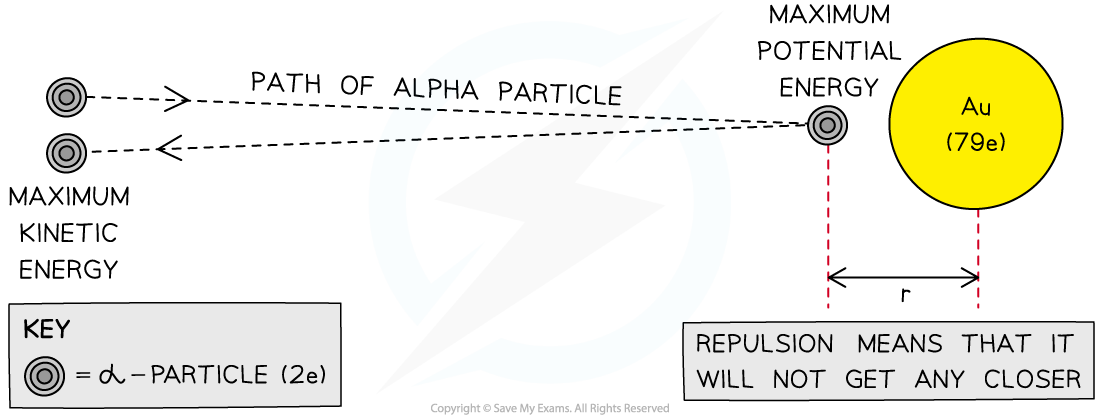
In the Rutherford scattering experiment, alpha particles are fired at a thin gold foil
Some of the alpha particles are found to come straight back from the gold foil
This indicates that there is electrostatic repulsion between the alpha particles and the gold nucleus

At the point of closest approach, r, the repulsive force reduces the speed of the alpha particles to zero momentarily
At this point, the initial kinetic energy of an alpha particle, Ek, is equal to electric potential energy, Ep
The radius of the closest approach can be found be equating the initial kinetic energy to the electric potential energy

Equating the two equations gives:

Examiner Tips and Tricks
It is important to understand that the nucleus is tiny compared to the size of the atom around it.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
