Coulomb's Law (OCR A Level Physics) : Revision Note
Coulomb's Law
All charged particles produce an electric field around it
This field exerts a force on any other charged particle within range
The electrostatic force between two charges is defined by Coulomb’s Law
Recall that the charge of a uniform spherical conductor can be considered as a point charge at its centre
Coulomb’s Law states that:
The electrostatic force between two point charges is proportional to the product of the charges and inversely proportional to the square of their separation
According to Coulomb's law, the electrostatic force between two point charges is calculated as follows:
Where:
F = electrostatic force (N)
Q, q = magnitudes of the charges (C)
r = distance between the centres of the two charges (m)
ε0 = permittivity of free space = 8.85 × 10–12 C2 N–1 m–2
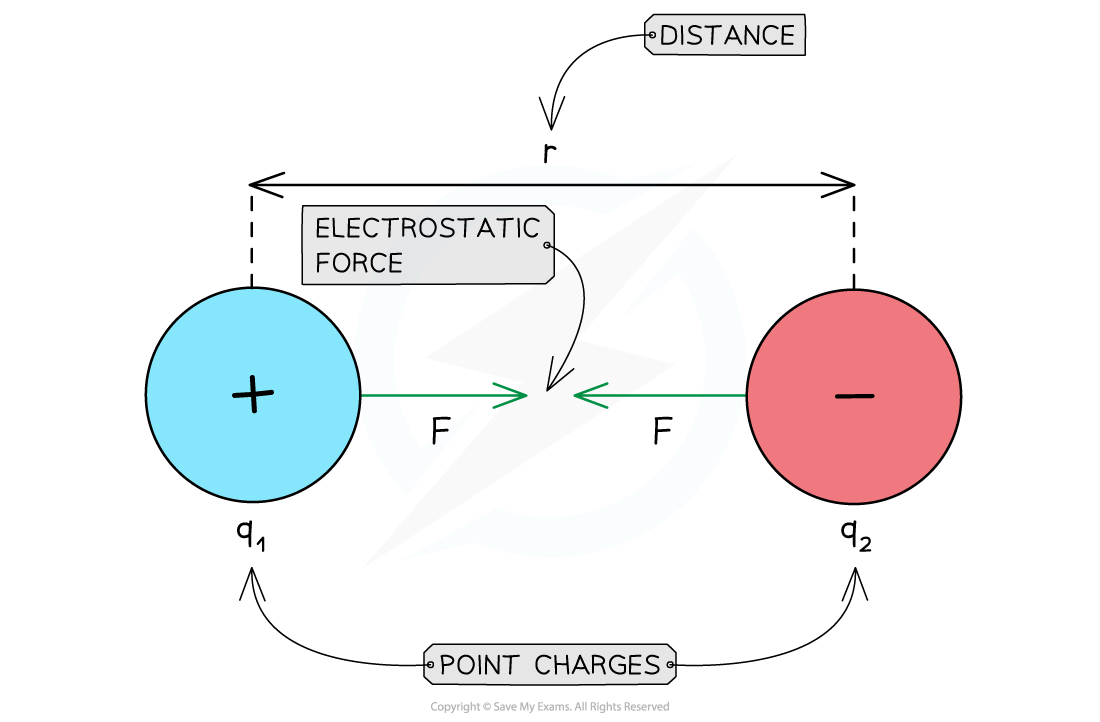
Attractive electrostatic force between two opposite charges
The 1/r2 relation is called the inverse square law
This means that when the separation of two charges doubles, the electrostatic force between them reduces by (½)2 = ¼
ε0 is the permittivity of free space
ε0 = 8.85 × 10–12 C2 N–1 m–2 and refers to charges in a vacuum
The value of the permittivity of air is taken to be the same as ε0
Any other material has a higher permittivity ε > ε0
ε is a measure of the resistance offered by a material in creating an electric field within it
Repulsive & Attractive Forces
For like charges:
The product Qq is positive
F is positive
The charges repel each other
For opposite charges:
The product Qq is negative
F is negative
The charges attract each other
Worked Example
An alpha particle is situated 2.0 mm away from a gold nucleus in a vacuum.
Assuming them to be point charges, calculate the magnitude of the electrostatic force acting on each of the charges.
Atomic number of helium = 2
Atomic number of gold = 79
Answer:
Step 1: Write down the known quantities
Distance, r = 2.0 mm = 2.0 × 10–3 m
Atomic number of helium = 2
Atomic number of gold = 79
Elementary charge, e = 1.60 × 10–19 C (from the data booklet)
Permettivity of free space, ε0 = 8.85 × 10–12 C2 N–1 m–2
Note: that you must convert the distance from millimetres (mm) into metres (m)
Step 2: Calculate the charges of the alpha particle and gold nucleus
The elementary charge e (when taken to be positive) is the charge of a proton
An alpha particle (helium nucleus) has 2 protons
So its charge is:
q = 2 × (1.60 × 10–19) = 3.2 × 10–19 C
A gold nucleus has 79 protons
So its charge is:
Q = 79 × (1.60 × 10–19) = 1.264 × 10–17 C
Step 3: Write down the equation to calculate the electrostatic force
Step 4: Substitute the numbers into the equation
F = 9.1 × 10–21 N
Examiner Tips and Tricks
You do not need to memorise the numerical value of the Coulomb's constant k or that of the permittivity of free space ε0. They will both be given in the data booklet.
Unless specified in the question, you should assume that charges are located in a vacuum.
You should note that Coulomb's law can only be applied to charged spheres whose size is much smaller than their separation. Only in this case, the point charge approximation is valid. You must remember that the separation r must be taken from the centres of the spheres.
You cannot use Coulomb's law to calculate the electrostatic force between charges distributed on irregularly-shaped objects.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
