The Avogadro Constant (OCR A Level Physics): Revision Note
Exam code: H556
Avogadro's Constant
The atomic mass unit (u) is approximately the mass of a proton or neutron = 1.66 × 10-27 kg
This means that the mass of an atom or molecule can be calculated using the number of protons and neutrons it contains
For example, a carbon−12 atom has a mass of:
12 u = 12 × 1.66 × 10-27 = 1.99 × 10-26 kg
The Mole
In thermodynamics, the amount of substance is measured in the SI unit ‘mole’
This has the symbol mol
The mole is a unit of substance, not a unit of mass
The mole is defined as:
The SI base unit of an ‘amount of substance’. It is the amount containing as many particles (e.g., atoms or molecules) as there are atoms in 12 g of carbon-12
The mole is an important unit in thermodynamics
If we consider the number of moles of two different gases under the same conditions, their physical properties are the same
One mole of a substance is defined as the number of molecules in exactly 12 g of carbon:
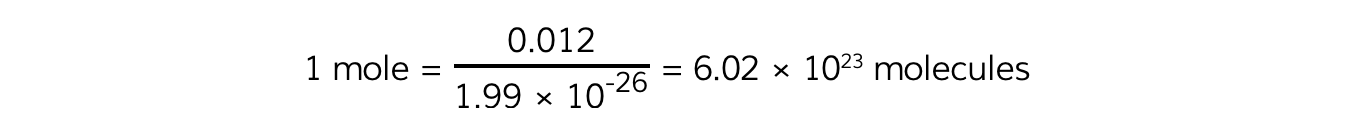
Avogadro's Constant
Avogadro’s constant (NA) is defined as:
The number of atoms of carbon-12 in 12 g of carbon-12; equal to 6.02 × 1023 mol−1
For example, 1 mole of sodium (Na) contains 6.02 × 1023 atoms of sodium
The number of atoms can be determined if the number of moles is known by multiplying by NA.
For example: 2.0 mol of nitrogen contains: 2.0 × NA = 2.0 × 6.02 × 1023 = 1.20 × 1024 atoms
Molar Mass
The molar mass of a substance is the mass, in grams, in one mole
Its unit is g mol−1
The number of moles from this can be calculated using the equation:
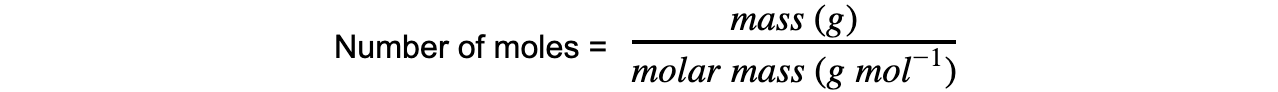

Unlock more, it's free!
Was this revision note helpful?
