Continuous, Emission Line & Absorption Line Spectrum (OCR A Level Physics) : Revision Note
Continuous, Emission Line & Absorption Line Spectrum
There are three kinds of light spectra:
Continuous emission spectra
Emission line spectra
Absorption line spectra
Continuous Line Spectra
Photons emitted from the core of a star contain all the wavelengths and frequencies of the electromagnetic spectrum
This is called a continuous spectrum
Continuous spectra are produced from hot, dense sources, such as the cores of stars

A continuous spectrum is one which emits all wavelengths of light
Emission Line Spectra
When an electron transitions from a higher energy level to a lower energy level, this results in the emission of a photon
Each transition corresponds to a different wavelength of light and this corresponds to a line in the spectrum
The resulting emission spectrum contains a set of discrete wavelengths
It is represented by coloured lines on a black background
Emission line spectra are produced by hot, low pressure gases

The emission spectrum of hydrogen
Absorption Spectra
An atom can be raised to an excited state by the absorption of a photon
When white light passes through a cool, low pressure gas it is found that light of certain wavelengths are missing
This type of spectrum is called an absorption spectrum
An absorption spectrum consists of a continuous spectrum
This is represented by a background of all the colours with dark lines at certain wavelengths
These dark lines correspond exactly to the differences in energy levels in an atom
When these electrons return to lower levels, the photons are emitted in all directions, rather than in the original direction of the white light
Therefore, some wavelengths appear to be missing
The wavelengths missing from an absorption spectrum are the same as their corresponding emission spectra of the same element
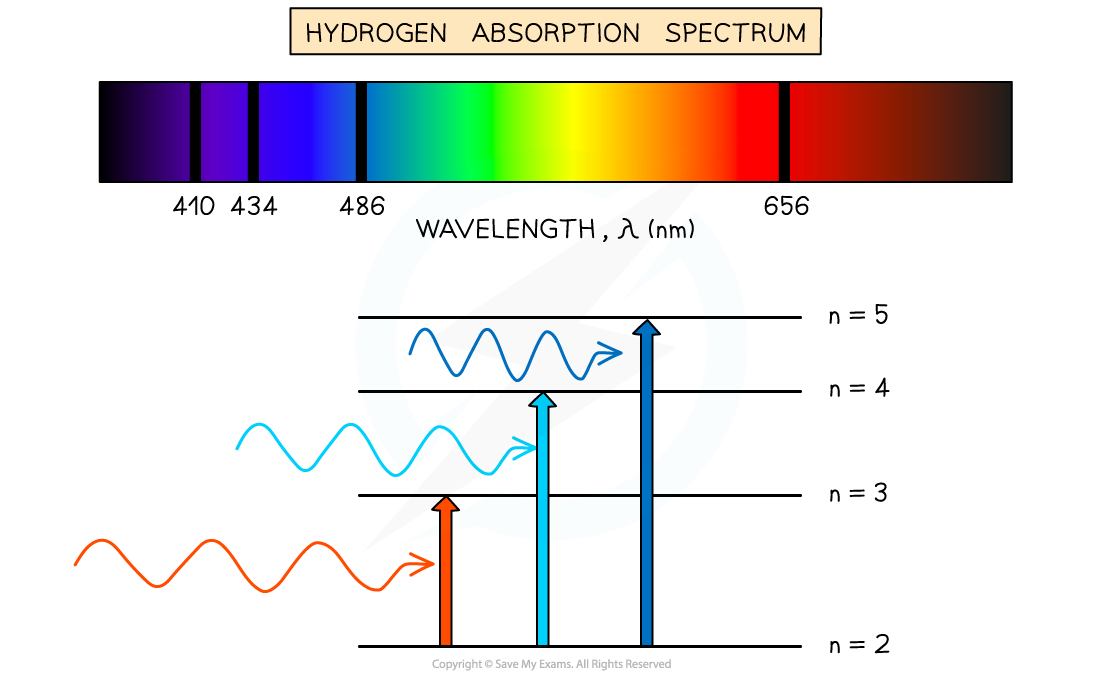
The absorption spectrum of hydrogen


Worked Example
An absorption line at a wavelength of 588 nm is observed in the spectrum from a star.
Determine the difference between the energy levels for the atoms in the gas responsible for this absorption line. Give your answer in electron volts.
Answer:
Step 1: List the known quantities
Wavelength, λ = 588 nm = 5.88 × 10–7 m
Planck’s constant, h = 6.63 × 10–34 J s
Speed of light, c = 3.00 × 108 m s–1
Step 2: Identify the appropriate equation
ΔE =
Step 3: Substitute the known values and calculate ΔE
ΔE = = 3.38 × 10–19 J
Step 4: Convert from J to eV
ΔE = = 2.11 eV
Worked Example
Explain why:
a) Hot, dense sources produce continuous spectra
b) Hot, low pressure gases produce emission spectra
c) Hot, dense sources observed through cool, low pressure gases produce absorption spectra
Answer:
Part (a)
Hot, dense sources, such as the cores of stars, produce continuous spectra because:
In a hot, dense material, the atoms or molecules are so close together that they interact with one another
This leads to a spread of energy states that are not clearly defined
Therefore, photons of all frequencies are emitted leading to an uninterrupted band of colour
Part (b)
Hot, low pressure gases produce emission line spectra, because:
Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas
The line spectrum has certain, fixed frequencies related to the differences in energy between the various energy levels of the atoms of the gas
In a low pressure gas, the atoms or molecules are not close together
This means the energy levels of the gas atoms or molecules are clearly quantised and well-defined
Therefore, only photons which correspond to the differences in energy between the energy levels of a bound electron are seen
Part (c)
Hot, dense sources observed through cold gases produce absorption spectra because:
Atoms of different elements in the cold gas absorb energy emitted from the hot source but only at particular energy values
These particular energy values correspond to the differences in energy between the energy levels of a bound electron
This means that particular frequencies of light are absorbed, creating black lines in the continuous emission spectrum
Examiner Tips and Tricks
You will be required to identify the part of the electromagnetic spectrum to which an absorption or an emission line belongs. To help with this, make sure you are familiar with the wavelength values.
You should also be able to determine the colour by knowing the wavelength when the absorption or emission lines are in the visible range.

You've read 1 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?

