Production & Use of X-rays (Cambridge (CIE) A Level Physics) : Revision Note
Production of X-rays
X-rays are short wavelength, high-frequency part of the electromagnetic spectrum
They have wavelengths in the range 10−8 to 10−13 m
X-rays are produced when fast-moving electrons rapidly decelerate and transfer their kinetic energy into photons of EM radiation
Producing X-rays
At the cathode (negative terminal), the electrons are released by thermionic emission
The electrons are accelerated towards the anode (positive terminal) at high speed
When the electrons bombard the metal target, they lose some of their kinetic energy by transferring it to photons
The electrons in the outer shells of the atoms (in the metal target) move into the spaces in the lower energy levels
As they move to lower energy levels, the electrons release energy in the form of X-ray photons
When an electron is accelerated, it gains energy equal to the electronvolt; this energy can be calculated using:
This is the maximum energy that an X-ray photon can have
Therefore, the maximum X-ray frequency fmax, or the minimum wavelength λmin, that can be produced is calculated using the equation:
Maximum frequency:
Minimum wavelength:
Where:
e = charge of an electron (C)
V = voltage across the anode (V)
h = Planck’s constant (J s)
c = speed of light (m s-1)
Worked Example
A typical spectrum of the X-ray radiation produced by electron bombardment of a metal target is shown below.
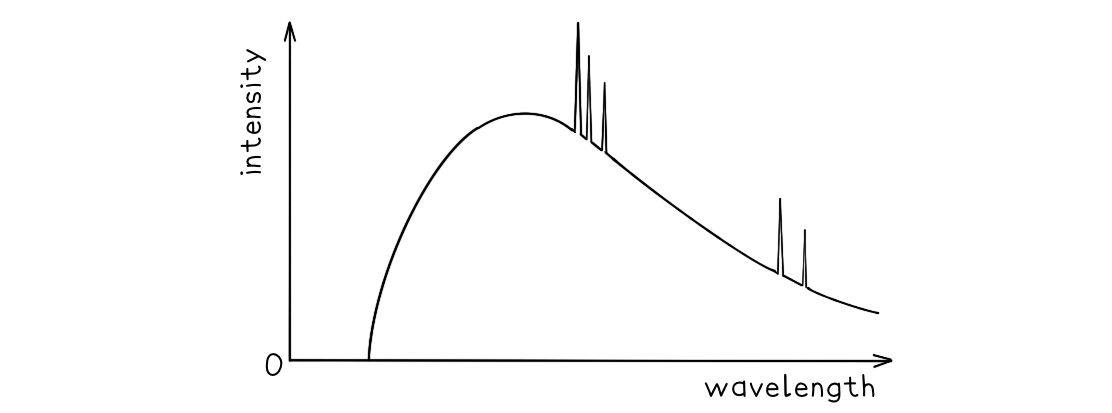
Explain why:
(a) a continuous spectrum of wavelengths is produced.
(b) the spectrum has a sharp cut-off at short wavelengths.
Answer:
Part (a)
Photons are produced whenever a charged particle is accelerated towards a metal target
The wavelength of the photons depends on the magnitude of the acceleration
The electrons which hit the target have a distribution of accelerations, therefore, a continuous spectrum of wavelengths is observed
Part (b)
The minimum wavelength is equal to
This equation shows the maximum energy of the electron corresponds to the minimum wavelength
Therefore, the higher the acceleration, the shorter the wavelength
At short wavelengths, the sharp cut-off occurs as each electron produces a single photon, so, all the electron energy is given up in one collision
Using X-rays in medical imaging
X-rays have been highly developed to provide detailed images of soft tissue and even blood vessels
When treating patients, the aims are to:
reduce the exposure to radiation as much as possible
improve the contrast of the image
Reducing exposure
X-rays are ionising, meaning they can cause damage to living tissue and can potentially lead to cancerous mutations
Therefore, healthcare professionals must ensure patients receive the minimum dosage possible
In order to do this, aluminium filters are used
This is because many wavelengths of X-ray are emitted
Longer wavelengths of X-ray are more penetrating, therefore, they are more likely to be absorbed by the body
This means they do not contribute to the image and pose more of a health hazard
The aluminium sheet absorbs these long wavelength X-rays making them safer
Contrast and sharpness
Contrast is defined as:
The difference in the degree of blackening between structures
Contrast allows a clear difference between tissues to be seen
Image contrast can be improved by:
using the correct level of X-ray hardness: hard X-rays for bones, soft X-rays for tissue
using a contrast media
Sharpness is defined as:
How well-defined the edges of structures are
Image sharpness can be improved by:
using a narrower X-ray beam
reducing X-ray scattering by using a collimator or lead grid
smaller pixel size

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
