Nuclear Equations (Cambridge (CIE) A Level Physics): Revision Note
Exam code: 9702
Representing simple nuclear reactions
A nucleus can be described using
notation
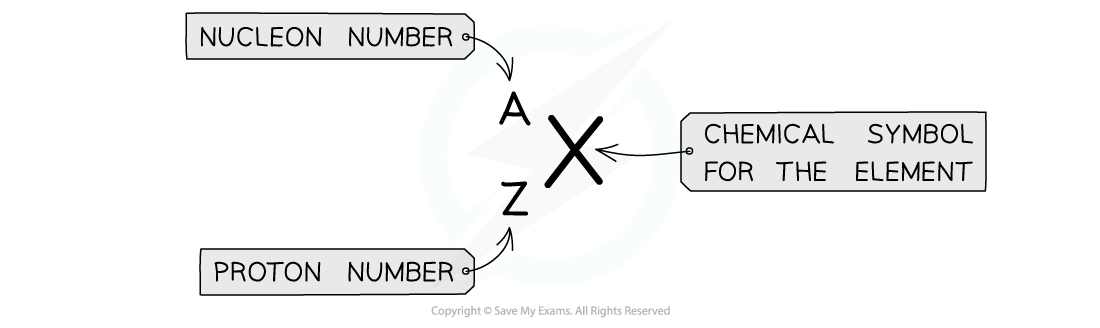
notation is used to describe the constituents of a nucleus
The top number A represents the nucleon number or the mass number
Nucleon number (A) = total number of protons and neutrons in the nucleus
The lower number Z represents the proton or atomic number
Proton number (Z) = total number of protons in the nucleus
Worked Example
When a neutron is captured by a uranium-235 nucleus, the outcome may be represented by the nuclear equation:
Determine the value of x.
Answer:
Step 1: Balance the nucleon numbers (the top number)
235 + 1 = 95 + 139 + x(1) + 7(0)
Step 2: Rearrange to find the value of x
x = 235 + 1 − 95 − 139 = 2

Unlock more, it's free!
Did this page help you?