Calculating Discrete Energies (Cambridge (CIE) A Level Physics): Revision Note
Exam code: 9702
Calculating discrete energies
The difference between two energy levels is equal to a specific photon energy
This difference is energy is calculated by the equation:
Where,
E1 = energy of the lower level (J)
E2 = energy of the higher level (J)
h = Planck’s constant (J s)
f = frequency of photon (Hz)
Using the wave equation, the wavelength of the emitted, or absorbed, radiation can be related to the energy difference by the equation:
This equation shows that the larger the difference in energy of two levels ΔE, the shorter the wavelength λ and vice versa
Worked Example
Some electron energy levels in atomic hydrogen are shown below.
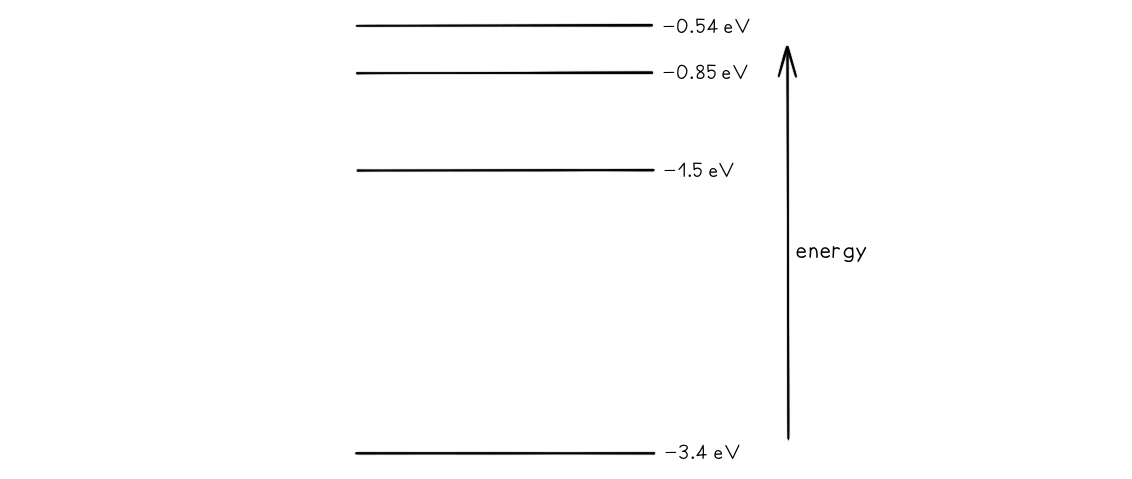
The longest wavelength produced as a result of electron transitions between two of the energy levels is 4.0 × 10–6 m.
a) Draw and mark:
The transition giving rise to the wavelength of 4.0 × 10–6 m with letter L.
The transition giving rise to the shortest wavelength with letter S.
b) Calculate the wavelength for the transition giving rise to the shortest wavelength.
Answer:
Part (a)
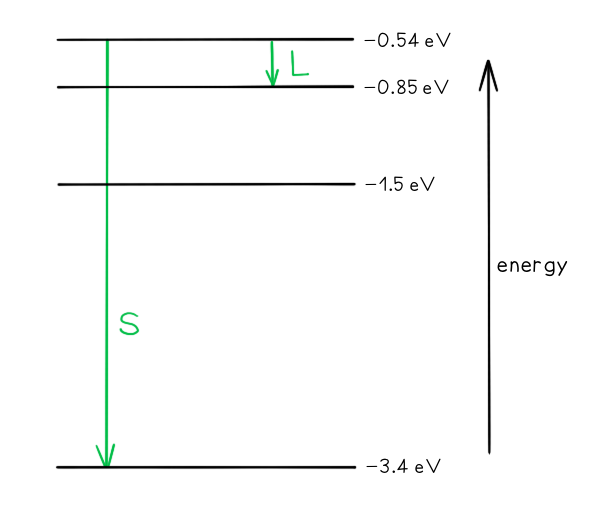
Photon energy and wavelength are inversely proportional, so the largest energy change corresponds to the shortest wavelength (line S) and the smallest energy change corresponds to the longest wavelength (line L)
Part (b)
Step 1: Write down the equation linking the wavelength and the energy levels
Step 2: Identify the energy levels giving rise to the shortest wavelength
E1 = –0.54 eV
E2 = –3.4 eV
Step 3: Calculate the wavelength
To convert from eV → J: multiply by 1.6 × 10-19
Examiner Tips and Tricks
Don't forget the minus sign and correctly know which energy level is E2 and E1.
The equation for ΔE gives the value of energy in joules. Therefore, if the energy has been given in eV, you have to convert this into joules for the calculations.
Although you may get a negative wavelength, the minus sign is not as important, but the value of the wavelength is for full marks.

Unlock more, it's free!
Was this revision note helpful?
