Atomic Energy Levels (Cambridge (CIE) A Level Physics): Revision Note
Exam code: 9702
Atomic energy levels
Electrons in an atom can have only certain specific (discrete) energies
These energies are called electron energy levels
They can be represented as a series of stacked horizontal lines increasing in energy
Normally, electrons occupy the lowest energy level available, this is known as the ground state
Electrons can gain energy and move up the energy levels if they absorb energy by:
Collisions with other atoms or electrons
Absorbing a photon
A physical source, such as heat
This is known as excitation, and when electrons move up an energy level, they are said to be in an excited state
If the electron gains enough energy to be removed from the atom entirely, this is known as ionisation
When an electron returns to a lower energy state from a higher excited state, it releases energy in the form of a photon
Electron energy levels in atomic hydrogen
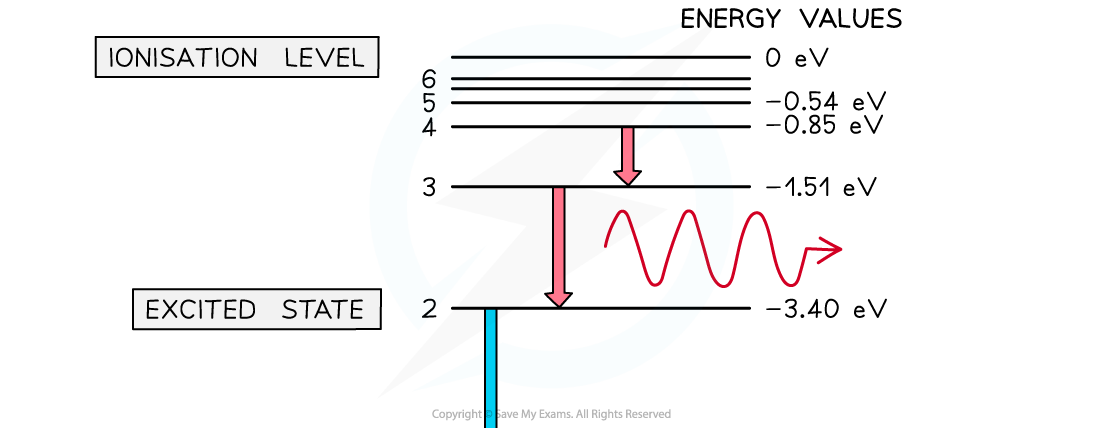

Photons are emitted when an electron moves from a higher energy state to a lower energy state

Unlock more, it's free!
Did this page help you?