Specific Heat Capacity (Cambridge (CIE) A Level Physics) : Revision Note
Defining specific heat capacity
The specific heat capacity of a substance is defined as:
The amount of thermal energy required to raise the temperature of 1 kg of a substance by 1 °C
This quantity determines the amount of energy needed to change the temperature of a substance
The specific heat capacity is measured in units of Joules per kilogram per Kelvin (J kg-1 K-1) or Joules per kilogram per Celsius (J kg-1 °C-1) and has the symbol c
Different substances have different specific heat capacities
Specific heat capacity is mainly used in liquids and solids
Specific heat capacity of different materials
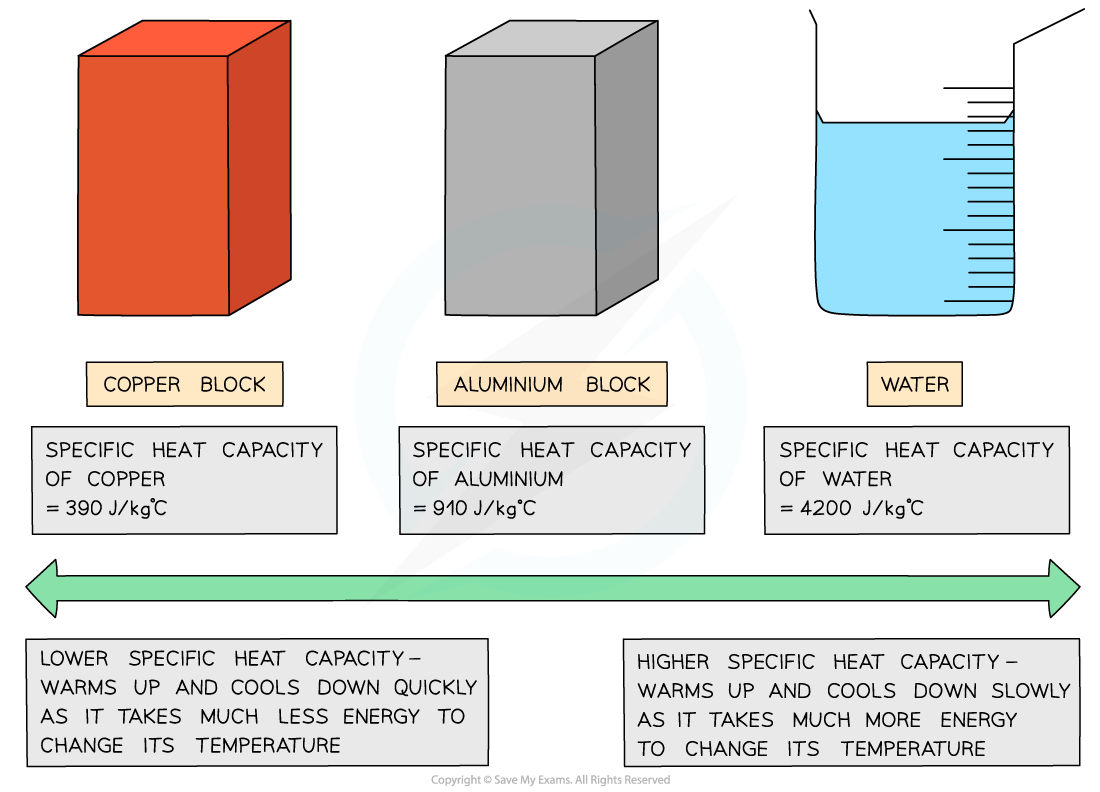
Low v high specific heat capacity.
From the definition of specific heat capacity, it follows that:
The heavier the material, the more thermal energy that will be required to raise its temperature
The larger the change in temperature, the higher the thermal energy will be required to achieve this change
Calculating specific heat capacity
The amount of thermal energy Q needed to raise the temperature by Δθ for a mass m with specific heat capacity c is equal to:
ΔQ = mcΔθ
Where:
ΔQ = change in thermal energy (J)
m = mass of the substance you are heating up (kg)
c = specific heat capacity of the substance (J kg-1K-1or J kg-1 °C-1)
Δθ = change in temperature (K or °C)
If a substance has a low specific heat capacity, it heats up and cools down quickly
If a substance has a high specific heat capacity, it heats up and cools down slowly
The specific heat capacity of different substances determines how useful they would be for a specific purpose eg. choosing the best material for kitchen appliances
Table of values of specific heat capacity for various substances
Substance | Specific heat capacity / J kg-1K-1 |
|---|---|
Aluminium | 910 |
Copper | 390 |
Lead | 126 |
Glass | 500 - 680 |
Water | 4200 |
Mercury | 140 |
Good electrical conductors, such as copper and lead, are also excellent conductors of heat due to their low specific heat capacity
Worked Example
A kettle is rated at 1.7 kW. A mass of 650 g of a liquid at 25°C is poured into a kettle. When the kettle is switched on, it takes 3.5 minutes to start boiling.
Calculate the specific heat capacity of the liquid.
Step 1: Calculate the Energy from the power and time
Energy = Power × Time
Power = 1.7 kW = 1.7 × 103 W
Time = 3.5 minutes = 3.5 × 60 = 210 s
Energy = 1.7 × 103 × 210 = 3.57 × 105 J
Step 2: Thermal energy equation
ΔQ = mcΔθ
Step 3: Rearrange for specific heat capacity
Step 4: Substitute in values
m = 650 g = 650 × 10-3 kg
Δθ = 100 - 25 = 75oC
= 7323.07... = 7300 J kg-1 oC-1 (2 s.f)
Examiner Tips and Tricks
The difference in temperature Δθ will be exactly the same whether the temperature is given in Celsius or Kelvin. Therefore, there is no need to convert between the two since the difference in temperature will be the same for both units.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
