Syllabus Edition
First teaching 2023
First exams 2025
Mass Defect & Binding Energy (Cambridge (CIE) A Level Physics): Revision Note
Mass defect & binding energy
Mass defect
Experiments into nuclear structure have found that the total mass of a nucleus is less than the sum of the masses of its constituent nucleons
This difference in mass is known as the mass defect
Mass defect is defined as:
The difference between the mass of a nucleus and the sum of the individual masses of its protons and neutrons
The mass defect Δm of a nucleus can be calculated using:
Δm = Zmp + (A – Z)mn – mtotal
Where:
Z = proton number
A = nucleon number
mp = mass of a proton (kg)
mn = mass of a neutron (kg)
mtotal = measured mass of the nucleus (kg)
Mass defect of carbon-12
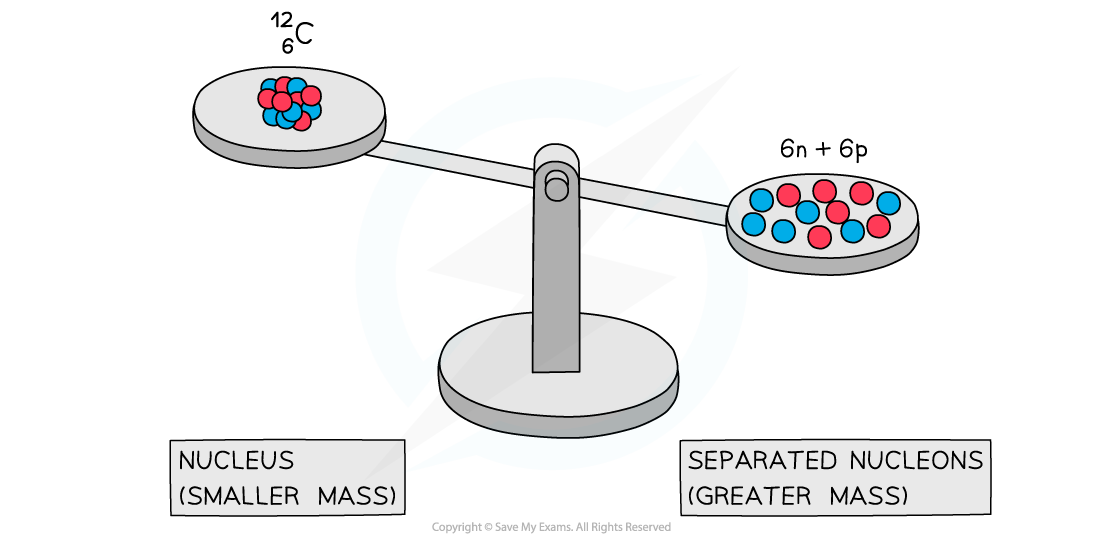
A system of separated nucleons has a greater mass than a system of bound nucleons
Due to the equivalence of mass and energy, this decrease in mass implies that energy is released in the process
Since nuclei are made up of neutrons and protons, there are forces of repulsion between the positive protons
Therefore, it takes energy, ie. the binding energy, to hold nucleons together as a nucleus
Binding energy
Binding energy is defined as:
The energy required to break a nucleus into its constituent protons and neutrons
Energy and mass are proportional, so, the total energy of a nucleus is less than the sum of the energies of its constituent nucleons
The formation of a nucleus from a system of isolated protons and neutrons is therefore an exothermic reaction
This means that it releases energy
This energy can be calculated using the equation:
E = Δmc2
In a typical nucleus, binding energies are usually measured in MeV
This is considerably larger than the few eV associated with the binding energy of electrons in the atom
Nuclear reactions involve changes in the nuclear binding energy whereas chemical reactions involve changes in the electron binding energy
This is why nuclear reactions produce much more energy than chemical reactions
Worked Example
Calculate the binding energy per nucleon, in MeV, for the radioactive isotope potassium-40 (19K).
Nuclear mass of potassium-40 = 39.953 548 u
Mass of one neutron = 1.008 665 u
Mass of one proton = 1.007 276 u
Answer:
Step 1: Identify the number of protons and neutrons in potassium-40
Proton number, Z = 19
Neutron number, N = 40 – 19 = 21
Step 2: Calculate the mass defect, Δm
Proton mass, mp = 1.007 276 u
Neutron mass, mn = 1.008 665 u
Mass of K-40, mtotal = 39.953 548 u
Δm = Zmp + Nmn – mtotal
Δm = (19 × 1.007276) + (21 × 1.008665) – 39.953 548
Δm = 0.36666 u
Step 3: Convert from u to kg
1 u = 1.661 × 10–27 kg
Δm = 0.36666 × (1.661 × 10–27) = 6.090 × 10–28 kg
Step 4: Write down the equation for mass-energy equivalence
E = Δmc2
Where c = speed of light
Step 5: Calculate the binding energy, E
E = 6.090 × 10–28 × (3.0 × 108)2 = 5.5 × 10–11 J
Step 6: Determine the binding energy per nucleon and convert J to MeV
Take the binding energy and divide it by the number of nucleons
1 MeV = 1.6 × 10–13 J
binding energy per nucleon = (5.5 × 10–11)/40 = 1.375 × 10–12 J
binding energy per nucleon = (1.375 × 10–12)/(1.6 × 10–13) = 8.594 MeV
Examiner Tips and Tricks
The terms binding energy and mass defect can cause students confusion, so be careful when using them in your explanations.
Avoid describing the binding energy as the energy stored in the nucleus – this is not correct – it is energy that must be put into the nucleus to separate all the nucleons.
The same goes for the term mass defect, make sure to only use this when all the nucleons are separated and not to describe the decrease in mass which occurs during radioactive decay.
Binding energy per nucleon
In order to compare nuclear stability, it is more useful to look at the binding energy per nucleon
The binding energy per nucleon is defined as:
The binding energy of a nucleus divided by the number of nucleons in the nucleus
A higher binding energy per nucleon indicates a higher stability
In other words, more energy is required to separate the nucleons contained within a nucleus
Graph of binding energies for nuclei of different masses
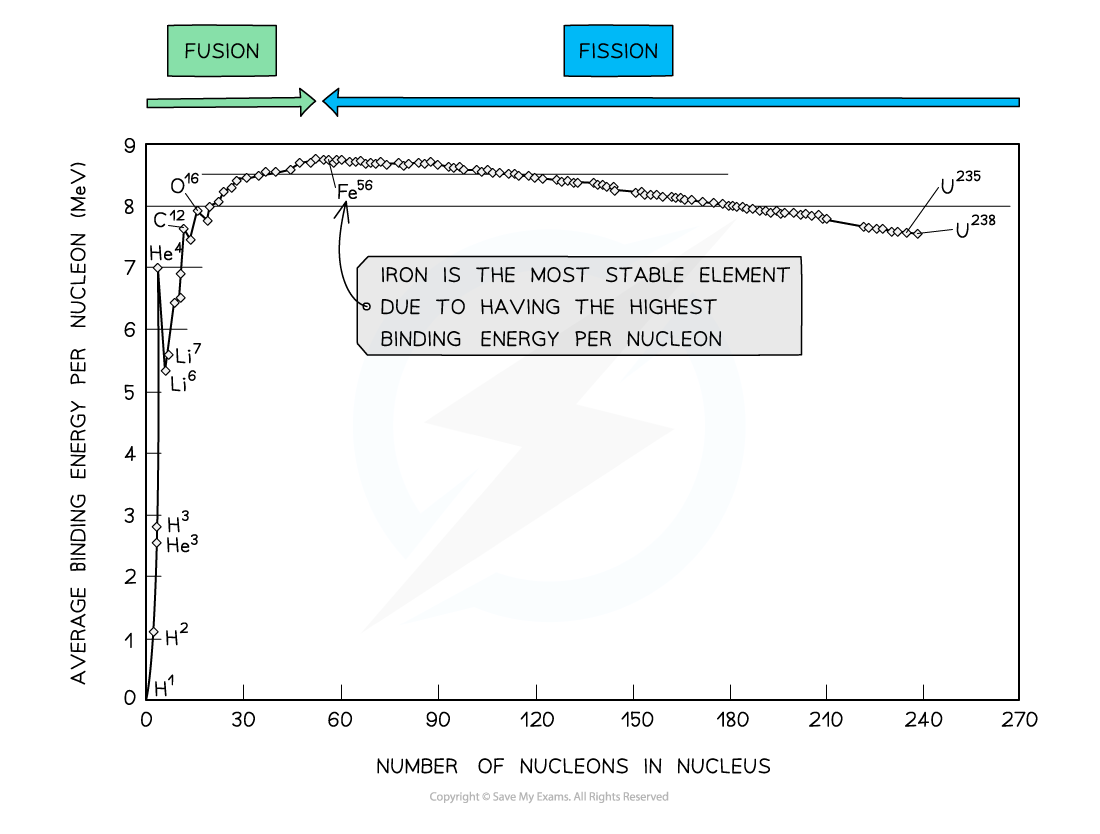
By plotting a graph of binding energy per nucleon against nucleon number, the stability of elements can be inferred
Key features of the graph
At low values of A:
Nuclei have lower binding energies per nucleon than at large values of A, but they tend to be stable when N = Z
This means light nuclei have weaker electrostatic forces and will undergo fusion
The gradient is much steeper compared to the gradient at large values of A
This means that fusion reactions release a greater binding energy than fission reactions
At high values of A:
Nuclei have generally higher binding energies per nucleon, but this gradually decreases with A
This means the heaviest elements are the most unstable and will undergo fission
The gradient is less steep compared to the gradient at low values of A
This means that fission reactions release less binding energy than fission reactions
Iron (A = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements
Helium (4He), carbon (12C) and oxygen (16O) do not fit the trend
Helium-4 is a particularly stable nucleus hence it has a high binding energy per nucleon
Carbon-12 and oxygen-16 can be considered to be three and four helium nuclei, respectively, bound together
Worked Example
Determine the binding energy per nucleon of iron-56, , in MeV.
Mass of a neutron = 1.675 × 10-27 kg
Mass of a proton = 1.673 × 10-27 kg
Mass of an iron-56 nucleus = 9.288 × 10-26 kg
Answer:
Step 1: Calculate the mass defect
Number of protons, Z = 26
Number of neutrons, A – Z = 56 – 26 = 30
Mass defect, Δm = Zmp + (A – Z)mn – mtotal
Δm = (26 × 1.673 × 10-27) + (30 × 1.675 × 10-27) – (9.288 × 10-26)
Δm = 8.680 × 10-28 kg
Step 2: Calculate the binding energy of the nucleus
Binding energy, E = Δmc2
E = (8.680 × 10-28) × (3.00 × 108)2 = 7.812 × 10-11 J
Step 3: Calculate the binding energy per nucleon
Step 4: Convert to MeV
J → eV: divide by 1.6 × 10-19
eV → MeV: divide by 106
Examiner Tips and Tricks
Checklist on what to include (and what not to include) in an exam question asking you to draw a graph of binding energy per nucleon against nucleon number:
You will be expected to draw the best fit curve AND a cross to show the anomaly that is helium
Do not begin your curve at A = 0, this is not a nucleus!
Make sure to correctly label both axes AND units for binding energy per nucleon
You will be expected to include numbers on the axes, mainly at the peak to show the position of iron (56Fe)

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
