The Work Function (Cambridge (CIE) A Level Physics): Revision Note
Exam code: 9702
The photoelectric equation
Since energy is always conserved, the energy of an incident photon is equal to:
The threshold energy + the kinetic energy of the photoelectron
The energy within a photon is equal to hf
This energy is transferred to the electron to release it from a material (the work function) and gives the emitted photoelectron the remaining amount as kinetic energy
This equation is known as the photoelectric equation:
Where:
h = Planck's constant (J s)
f = the frequency of the incident radiation (Hz)
hf = energy of a single photon (J)
Φ = the work function of the material (J)
½mv2max= the maximum kinetic energy of the photoelectrons (J)
This is always written as Ek max
This equation demonstrates:
If the incident photons do not have a high enough frequency (f) and energy to overcome the work function (Φ), then no electrons will be emitted
Above energy hf0 = Φ, where f0 = threshold frequency, photoelectric emission can occur
Ekmax depends only on the frequency of the incident photon, and not the intensity of the radiation
The majority of photoelectrons will have kinetic energies less than Ekmax
Graphical representation of work function
The photoelectric equation can be rearranged into the straight-line equation:
Comparing this to the photoelectric equation:
A graph of maximum kinetic energy Ekmax against frequency f can be obtained
Graph of kinetic energy against frequency

The graph of maximum kinetic energy of photoelectrons against photon frequency is straight line with an x-intercept
The key elements of the graph are:
The work function Φ is the y-intercept
The threshold frequency f0 is the x-intercept
The gradient is equal to Planck's constant h
There are no electrons emitted below the threshold frequency f0
Worked Example
The graph below shows how the maximum kinetic energy Ek of electrons emitted from the surface of sodium metal varies with the frequency f of the incident radiation.
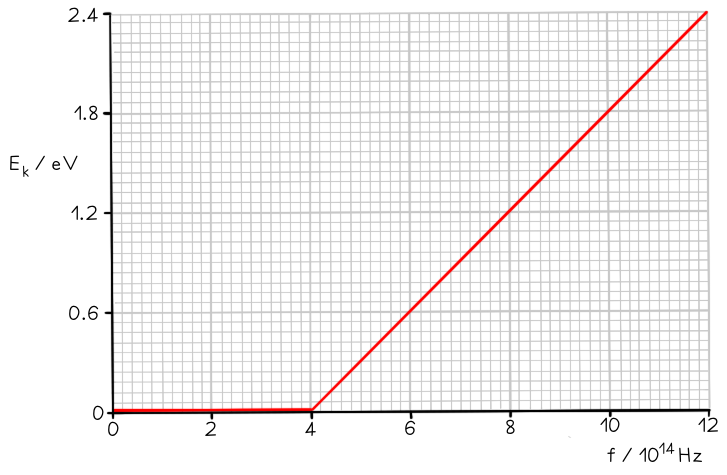
Calculate the work function of sodium in eV.
Answer:
Step 1: Write out the photoelectric equation and rearrange it to fit the equation of a straight line
Step 2: Identify the threshold frequency from the x-axis of the graph
When Ek = 0, f = f0
Therefore, the threshold frequency is f0 = 4 × 1014 Hz
Step 3: Calculate the work function
From the graph at f0, Ek = 0
Step 4: Convert the work function into eV
1 eV = 1.6 × 10-19 J
Examiner Tips and Tricks
You must recognise how to compare linear equations to the straight-line equation y = mx + c. This is very common in A-level Physics!
Intensity & photoelectric current
Kinetic energy & intensity
The kinetic energy of the photoelectrons is independent of the intensity of the incident radiation
This is because each electron can only absorb one photon
Kinetic energy is only dependent on the frequency of the incident radiation
Intensity is the rate of energy transferred per unit area and is related to the number of photons striking the metal plate
Increasing the number of photons striking the metal will not increase the kinetic energy of the electrons; it will increase the number of photoelectrons emitted
Why the kinetic energy is a maximum
Each electron in the metal acquires the same amount of energy from the photons in the incident radiation for any given frequency
However, the energy required to remove an electron from the metal varies because some electrons are on the surface whilst others are deeper in the metal
The photoelectrons with the maximum kinetic energy will be those on the surface of the metal since they do not require much energy to leave the metal
The photoelectrons with lower kinetic energy are those deeper within the metal since some of the energy absorbed from the photon is used to approach the metal surface (and overcome the work function)
There is less kinetic energy available for these photoelectrons once they have left the metal
Photoelectric current
Current is defined as the flow of charge, in this case, photoelectrons
The photoelectric current is a measure of the number of photoelectrons emitted per second
The value of the photoelectric current is calculated by the number of electrons emitted multiplied by the charge on one electron
Photoelectric current is proportional to the intensity of the radiation incident on the surface of the metal
This is because intensity is proportional to the number of photons striking the metal per second
Since each photoelectron absorbs a single photon, the photoelectric current must be proportional to the intensity of the incident radiation

Sketch graphs showing the trends in the variation of electron KE with the frequency and intensity of the incident light and the variation of photocurrent with the intensity of the incident light
Examiner Tips and Tricks
If you change the frequency of the incident light whilst keeping the number of photons emitted from the light source constant, then the photoelectric current will remain constant
This is because changing the frequency will change the energy of the emitted photons, but the number of photons will remain the same
If you change the frequency of the incident light whilst keeping the intensity constant, then the photoelectric current will change
This is because intensity is power per unit area which is equal to the rate of energy transfer per unit area
The energy transferred comes from the photons, where the energy of a single photon is hf
So to account for n number of photons:
If the frequency, f, is increased and the intensity, , remains constant, then the number of photons, n, must decrease
Planck's constant, h, and the area, A, of the metal plate do not change
This is because at higher frequencies, each photon has a higher energy, so fewer photons are required to maintain the intensity

Unlock more, it's free!
Was this revision note helpful?
