Syllabus Edition
First teaching 2023
First exams 2025
The Photoelectric Effect: Basics (Cambridge (CIE) A Level Physics): Revision Note
The Photoelectric effect: basics
The photoelectric effect
The photoelectric effect is the phenomenon in which electrons are emitted from the surface of a metal upon the absorption of electromagnetic radiation
Electrons removed from a metal in this manner are known as photoelectrons
The emission of photoelectrons is known as photoelectric emission
The photoelectric effect provides important evidence that light is quantised
This is shown by the fact each electron can absorb only a single photon
This means only the frequencies of light above a threshold frequency will emit a photoelectron
The photoelectric effect
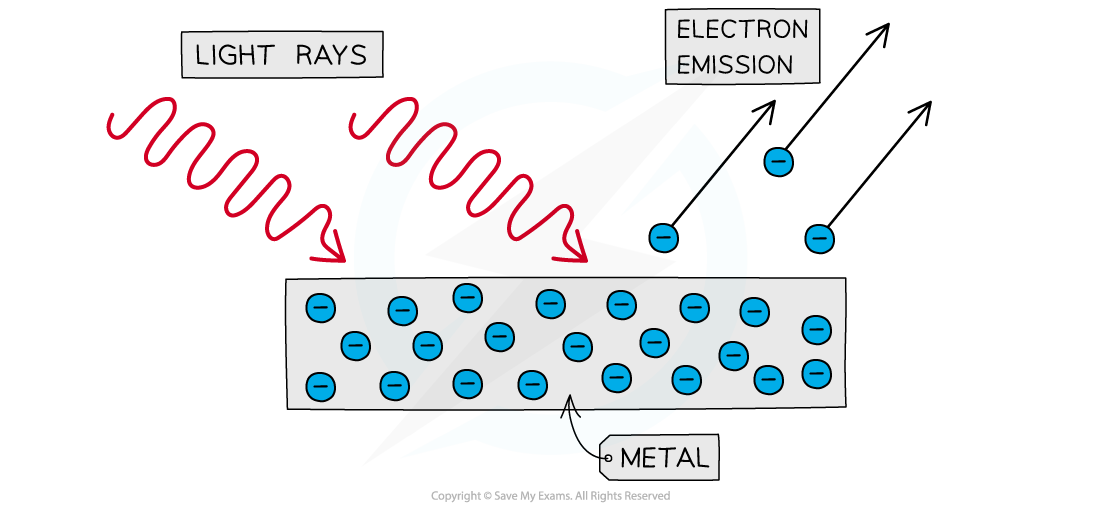
Photoelectrons are emitted from the surface of metal when light shines onto it
Examiner Tips and Tricks
Make sure to brush up on common misconceptions:
The electrons are already in the metal, they are not produced in any way by the incoming light rays
Each electron can absorb only one photon

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
