Average Kinetic Energy of a Molecule
- An important property of molecules in a gas is their average kinetic energy
- This can be deduced from the ideal gas equations relating pressure, volume, temperature and speed
- Recall the ideal gas equation:
pV = NkT
- Also recall the equation linking pressure and mean square speed of the molecules:

- The left hand side of both equations are equal (pV)
- This means the right hand sides are also equal:

- N will cancel out on both sides and multiplying by 3 obtains the equation:
m<c2> = 3kT
- Recall the familiar kinetic energy equation from mechanics:

- Instead of v2 for the velocity of one particle, <c2> is the average speed of all molecules
- Multiplying both sides of the equation by ½ obtains the average translational kinetic energy of the molecules of an ideal gas:

- Where:
- EK = kinetic energy of a molecule (J)
- m = mass of one molecule (kg)
- <c2> = mean square speed of a molecule (m2 s-2)
- k = Boltzmann constant
- T = temperature of the gas (K)
- Note: this is the average kinetic energy for only one molecule of the gas
- A key feature of this equation is that the mean kinetic energy of an ideal gas molecule is proportional to its thermodynamic temperature
EK ∝ T
- Translational kinetic energy is defined as:
The energy a molecule has as it moves from one point to another
- A monatomic (one atom) molecule only has translational energy, whilst a diatomic (two-atom) molecule has both translational and rotational energy
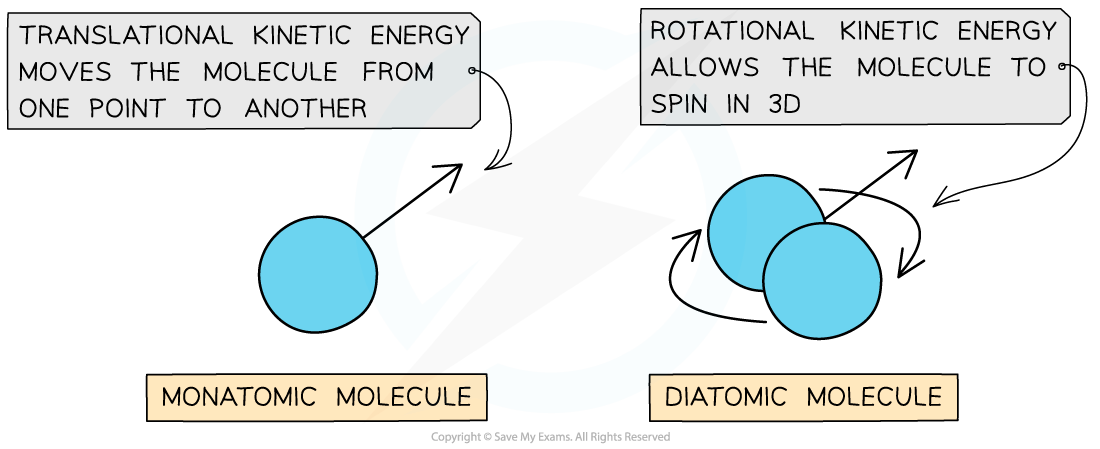
A diatomic molecule has both rotational and translational kinetic energy
Worked example
Helium can be treated like an ideal gas.Helium molecules have a root-mean-square (r.m.s) speed of 730 m s-1 at a temperature of 45 oC.
Calculate the r.m.s speed of the molecules at a temperature of 80 oC.
Step 1: Write down the equation for the average translational kinetic energy:

Step 2: Find the relation between cr.m.s and temperature T
Since m and k are constant, <c2> is directly proportional to T
<c2> ∝ T
Therefore, the relation between cr.m.s and T is:

Step 3: Write the equation in full

where a is the constant of proportionality
Step 4: Calculate the constant of proportionality from values given by rearranging for a:
T = 45 oC + 273.15 = 318.15 K

Step 5: Calculate cr.m.s at 80 oC by substituting the value of a and new value of T
T = 80 oC + 273.15 = 353.15 K

Examiner Tip
Keep in mind this particular equation for kinetic energy is only for one molecule in the gas. If you want to find the kinetic energy for all the molecules, remember to multiply by N, the total number of molecules.You can remember the equation through the rhyme ‘Average K.E is three-halves kT’.

