Nuclear Fusion & Fission (AQA A Level Physics)
Revision Note
Nuclear Fusion & Fission
Nuclear Fusion
Fusion is defined as:
The fusing together of two small nuclei to produce a larger nucleus
Low mass nuclei (such as hydrogen and helium) can undergo fusion and release energy
When two protons fuse, the element deuterium is produced
In the centre of stars, the deuterium combines with a tritium nucleus to form a helium nucleus, plus the release of energy, which provides fuel for the star to continue burning
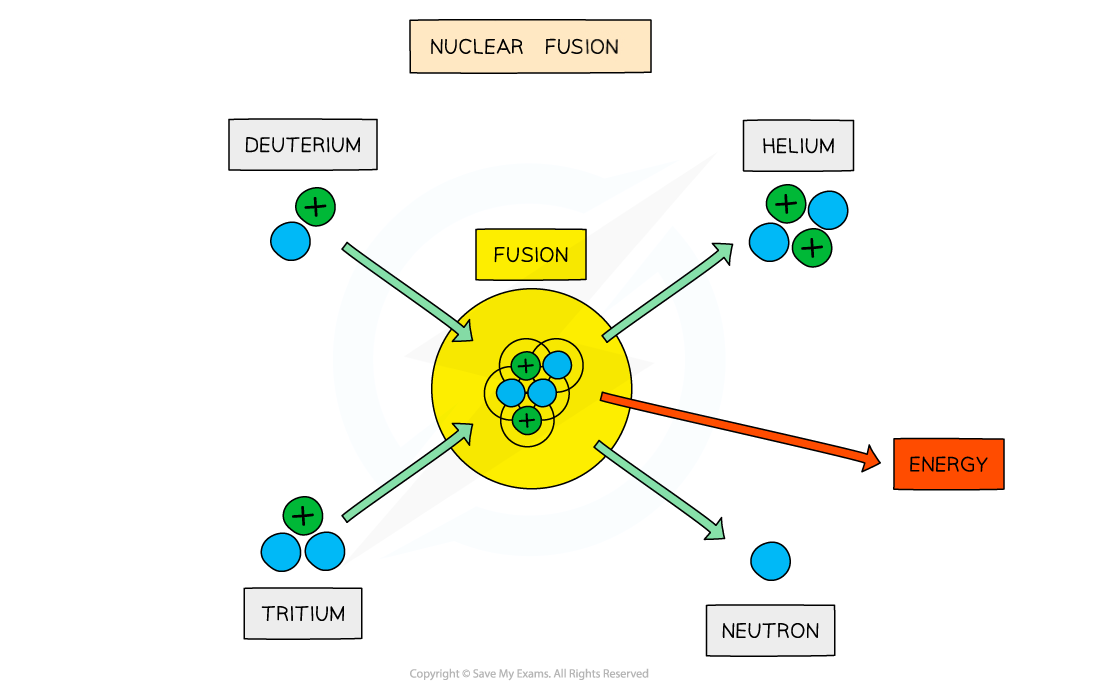
The fusion of deuterium and tritium to form helium with the release of energy
For two nuclei to fuse, both nuclei must have high kinetic energy
This is because nuclei must be able to get close enough to fuse
However, two forces acting within the nuclei make this difficult to achieve
Electrostatic Repulsion
Protons inside the nuclei are positively charged, which means that they electrostatically repel one another
Strong Nuclear Force
The strong nuclear force, which binds nucleons together, acts at very short distances within nuclei
Therefore, nuclei must get very close together for the strong nuclear force to take effect
It takes a great deal of energy to overcome the electrostatic force, hence fusion can only be achieved in an extremely hot environment, such as the core of a star
Nuclear Fission
Fission is defined as:
The splitting of a large atomic nucleus into smaller nuclei
High mass nuclei (such as uranium) can undergo fission and release energy
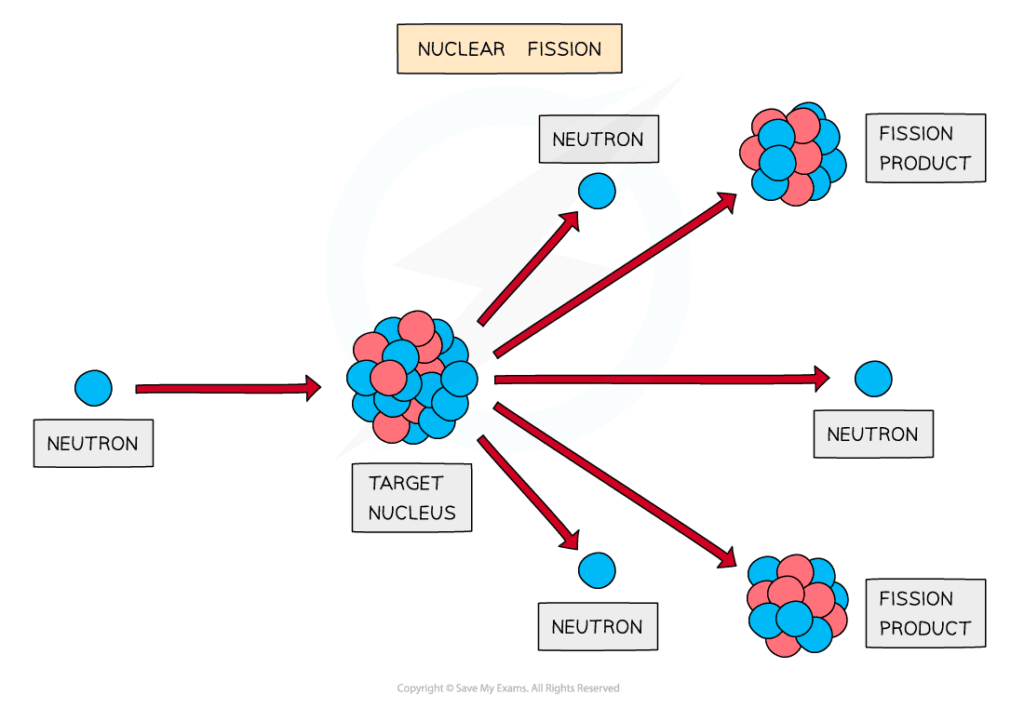
The fission of a target nucleus, such as uranium, to produce smaller daughter nuclei with the release of energy
Fission must first be induced by firing neutrons at a nucleus
When the nucleus is struck by a neutron, it splits into two, or more, daughter nuclei
During fission, neutrons are ejected from the nucleus, which in turn, can collide with other nuclei which triggers a cascade effect
This leads to a chain reaction which lasts until all of the material has undergone fission, or the reaction is halted by a moderator
Nuclear fission is the process which produces energy in nuclear power stations, where it is well controlled
When nuclear fission is not controlled, the chain reaction can cascade to produce the effects of a nuclear bomb
Examiner Tips and Tricks
When an atom undergoes nuclear fission, take note that extra neutrons are ejected by the nucleus and not from the fission products
Calculating Energy Released in Nuclear Reactions
The binding energy is equal to the amount of energy released in forming the nucleus, and can be calculated using:
E = (Δm)c2
Where:
E = Binding energy released (J)
Δm = mass defect (kg)
c = speed of light (m s-1)
The daughter nuclei produced as a result of both fission and fusion have a higher binding energy per nucleon than the parent nuclei
Therefore, energy is released as a result of the mass difference between the parent nuclei and the daughter nuclei
Worked Example
When a uranium-235 nucleus absorbs a slow-moving neutron and undergoes fission one possible pair of fission fragments is technetium-112 and indium-122. The equation for this process and the binding energy per nucleon for each isotope is shown below.

Binding energy per nucleon of U-235 = 7.59 MeV
Binding energy per nucleon of Tc-112 = 8.36 MeV
Binding energy per nucleon of In-122 = 8.51 MeV
When a uranium-235 nucleus undergoes fission in this way, calculate:
(a) The energy released, in MeV
(b) The mass defect
Answer:
Part (a)
Step 1: Determine the binding energies on each side of the equation
Binding energy = Binding Energy per Nucleon × Mass Number
Binding energy before (U) = 235 × 7.59 = 1784 MeV
Binding energy after (Tc + In) = (112 × 8.36) + (122 × 8.51) = 1975 MeV
Step 2: Find the difference between the energies
Energy released = 1975 – 1784 = 191 MeV
Part (b)
Method 1
Step 1: Convert the energy released from MeV to J
1 MeV = 1.60 × 10−13 J
Energy released = 191 × (1.60 × 10−13) = 3.06 × 10−11 J
Step 2: Write down the equation for mass-energy equivalence
E = Δmc2
Where c = speed of light
Step 3: Rearrange and determine the mass defect, Δm

Δm = 3.4 × 10−28 kg
Method 2
Step 1: Convert the energy released from MeV to u

Step 2: Calculate the mass defect, Δm
1 u = 1.66 × 10−27 kg
Δm = 0.205 × (1.66 × 10−27) = 3.4 × 10−28 kg
Examiner Tips and Tricks
Both methods for calculating mass defect are perfectly valid. It is highly recommended that you practice both ways and see which method you are most comfortable using.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
