Nuclear Density (AQA A Level Physics): Revision Note
Exam code: 7408
Constant Density of Nuclear Material
Assuming that the nucleus is spherical, its volume is equal to:
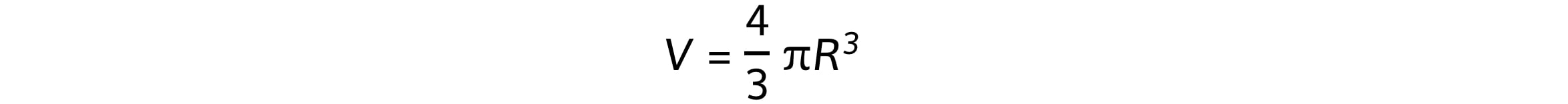
Where R is the nuclear radius, which is related to mass number, A, by the equation:
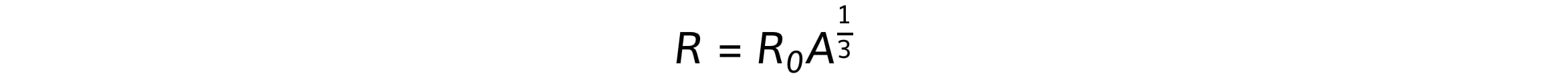
Where R0 is a constant of proportionality
Combining these equations gives:
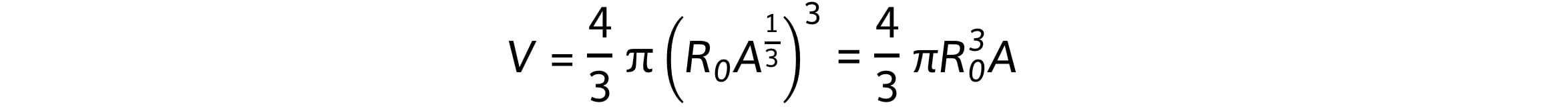
Therefore, the nuclear volume, V, is proportional to the mass of the nucleus, A
Mass (m), volume (V), and density (ρ) are related by the equation:
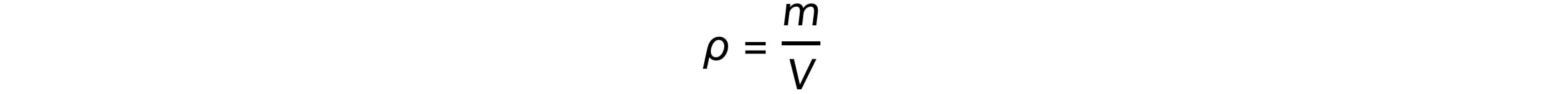
The mass, m, of a nucleus is equal to:
m = Au
Where:
A = the mass number
u = atomic mass unit
Using the equations for mass and volume, nuclear density is equal to:
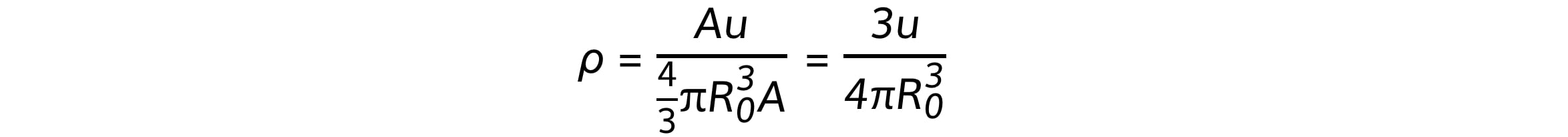
Since the mass number A cancels out, the remaining quantities in the equation are all constant
Therefore, this shows the density of the nucleus is:
Constant
Independent of the radius
The fact that nuclear density is constant shows that nucleons are evenly separated throughout the nucleus regardless of their size
Nuclear Density
Using the equation derived above, the density of the nucleus can be calculated:
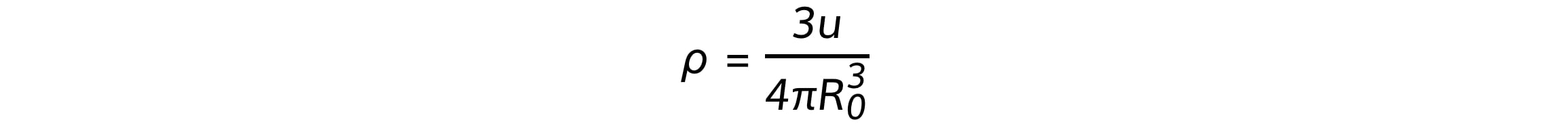
Where:
Atomic mass unit, u = 1.661 × 10–27 kg
Constant of proportionality, R0 = 1.05 × 10–15 m
Substituting the values gives a density of:
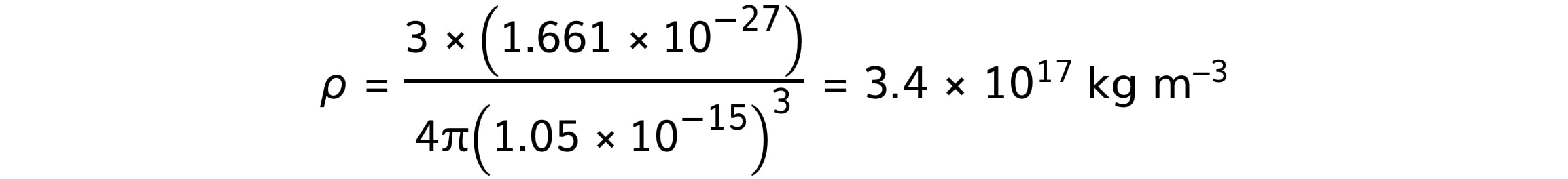
The accuracy of nuclear density depends on the accuracy of the constant R0, as a guide nuclear density should always be of the order 1017 kg m–3
Nuclear density is significantly larger than atomic density, this suggests:
The majority of the atom’s mass is contained in the nucleus
The nucleus is very small compared to the atom
Atoms must be predominantly empty space

Unlock more, it's free!
Did this page help you?