Applications of Radioactivity (AQA A Level Physics): Revision Note
Exam code: 7408
Applications of Radioactivity
Radioactivity has a wide variety of uses in industry, agriculture and medicine
Some of the main uses are
In medicine e.g. radiotherapy, tracers and sterilising equipment
Radiocarbon dating of archaeological artefacts
Uranium-lead dating of rock samples
Radioisotope power systems
Radiocarbon Dating
The isotope carbon-14 is commonly used in radioactive dating
It forms as a result of cosmic rays knocking out neutrons from nuclei, which then collide with nitrogen nuclei in the air:
All living organisms absorb carbon-14, but after they die they do not absorb any more
The proportion of carbon-14 is constant in living organisms as carbon is constantly being replaced during the period they are alive
When they die, the activity of carbon-14 in the organic matter starts to fall, with a half-life of around 5730 years
Samples of living material can be tested by comparing the current amount of carbon-14 in them and compared to the initial amount (which is based on the current ratio of carbon-14 to carbon-12), and hence they can be dated
Reliability of Carbon Dating
Carbon dating is a highly reliable method for estimating the ages of samples between 500 and 60 000 years old
This range can be explained by looking at the decay curve of carbon-14:
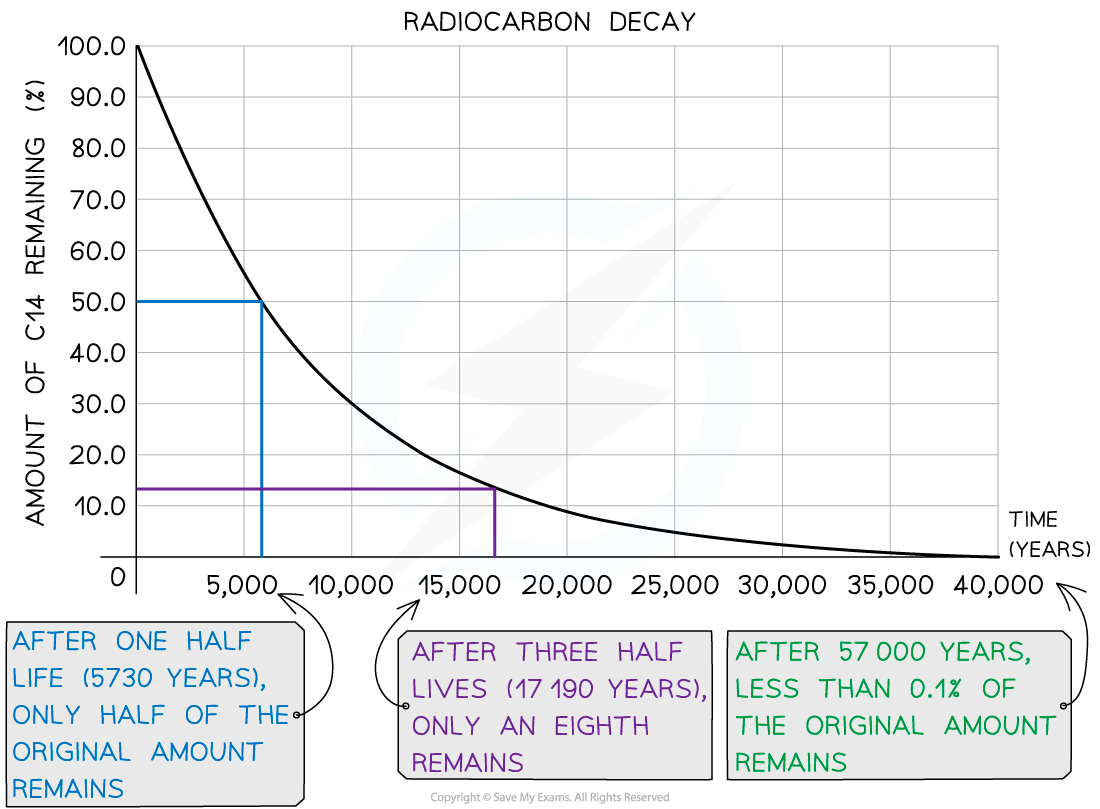
Carbon-14 decay curve used for radiocarbon dating
If the sample is less than 500 years old:
The activity of the sample will be too high to measure small changes accurately
Therefore, the ratio of carbon-14 to carbon-12 will be too high to determine an accurate age
If the sample is more than 60 000 years old:
The activity will be too low to distinguish between changes in the sample and background radiation
Therefore, the ratio of carbon-14 to carbon-12 will be too small to determine an accurate age
Uranium-Lead Dating
For many years, scientists could not agree on the age of the Earth
Until recently, the Earth was believed to be only millions of years old
Over the last century, radiometric dating methods have enabled scientists to discover the age of the Earth is many billions of years old
The most critical of these methods is uranium-lead dating
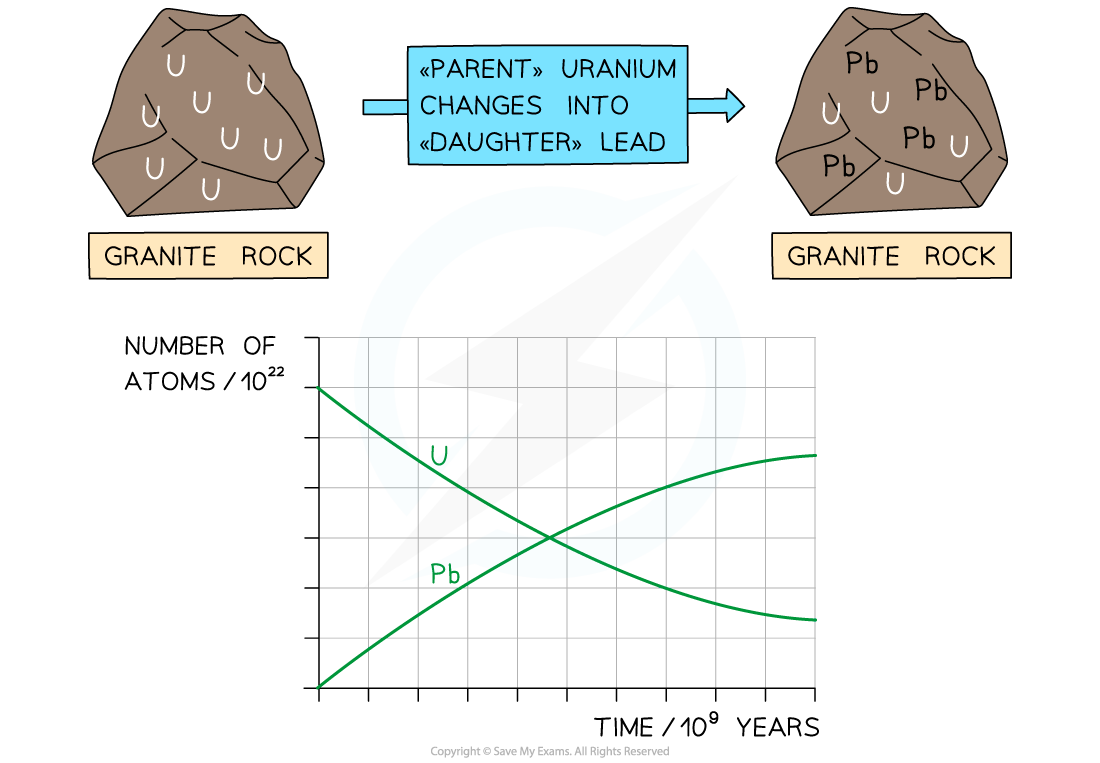
Uranium atoms decay whilst the number of lead atoms increases
Initially, there is only uranium in the rock, but over time, the uranium decays via a decay chain which ends with lead-206, which is a stable isotope
Uranium-238 has a half-life of 4.5 billion years
Over time, the ratio of lead-206 atoms to uranium-238 atoms increases
The ratio of uranium to lead in a sample of rock can then be used to determine its age
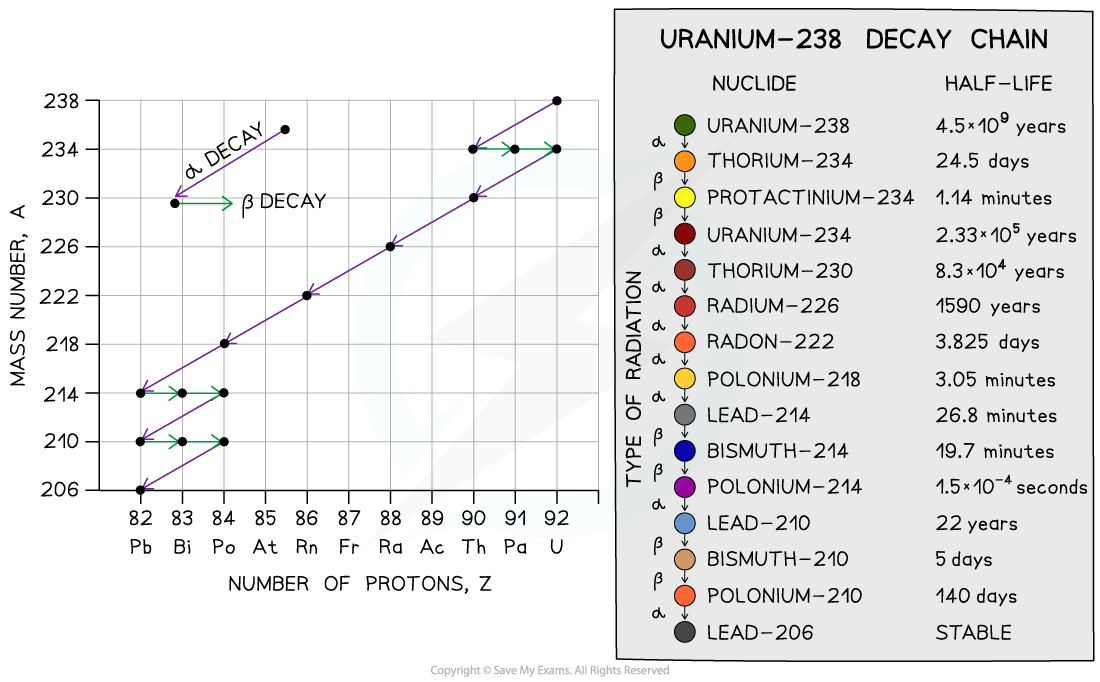
Uranium-238 decay chain
Radioisotope Power Systems
The decay of an isotope may release energy as heat
Radioisotopic power systems are designed to transform this heat into electrical power
Such devices have been used to power space probes and satellites
Typically, plutonium-238 is used as fuel, with 1 g generating a power output of about 500 mW
Worked Example
A space probe uses a source containing 4.0 kg of plutonium-238.
Plutonium-238 is an alpha-emitter with a half-life of 87.7 years. Each alpha decay releases 5.5 MeV per emission. The space probe converts this into electrical energy with an efficiency of 32%.
The space probe can continue to operate as long as the power output is maintained at 0.4 kW or above.
Estimate the time, in years, the source is expected to supply power to the space probe.
Answer:
Step 1: List the known quantities
Mass of Pu-238 = 4.0 kg = 4000 g
Molar mass of Pu-238 = 238 g mol−1
Avogadro's constant,
= 6.02 × 1023 mol−1
Half-life of Pu-238 = 87.7 years
Energy released per alpha decay = 5.5 MeV
1 electronvolt (eV) = 1.6 × 10−19 J
Efficiency = 32% = 0.32
Final power output, P = 0.4 kW = 400 W
Step 2: Calculate the initial number of nuclei present in the source
238 g of plutonium-238 contains 6.02 × 1023 atoms (Avogadro's number), so in 4 kg:
Number of nuclei:
Initial number of nuclei: nuclei
Step 3: Calculate the initial activity of the source
Decay constant:
Activity:
Combining these gives:
Initial activity:
Bq
Step 4: Calculate the initial power output of the source
Power output:
Energy released per decay:
Activity represents the decays per second, so:
Initial power output:
W
The electrical power transferred to the probe is:
W
Step 5: Use the exponential decay equation to calculate the time of operation
The power available is proportional to the activity of the isotope, so:
Exponential decay of power:
years
Therefore, the source is expected to supply power to the space probe for 73.3 years

Unlock more, it's free!
Did this page help you?