Half-Life (AQA A Level Physics): Revision Note
Exam code: 7408
Half-Life
Half-life is defined as:
The average time taken for a given number of nuclei of a particular isotope to halve
Since activity
is proportional to the number of undecayed nuclei
, the activity of the sample will also halve
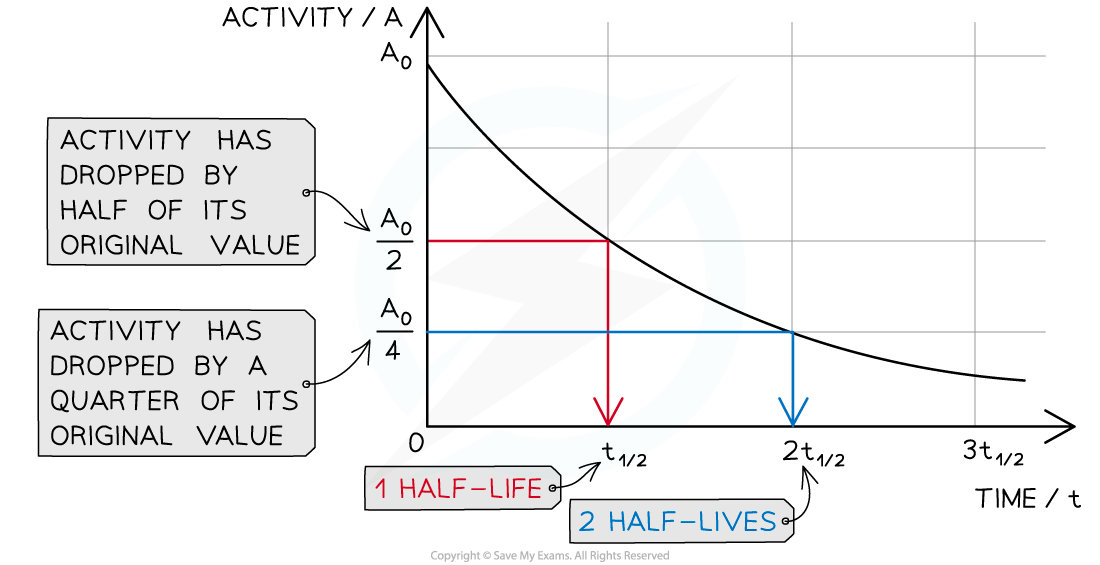
When a time equal to the half-life passes, the activity falls by half, when two half-lives pass, the activity falls by another half (which is a quarter of the initial value)
To find an expression for half-life, start with the equation for exponential decay:
Where:
N = number of nuclei remaining in a sample
N0 = the initial number of undecayed nuclei (when t = 0)
λ = decay constant (s-1)
t = time interval (s)
When time
is equal to the half-life
, the activity
of the sample will be half of its original value, so
The formula can then be derived by first, dividing both sides by
:
Then, taking the natural log of both sides:
Finally, applying properties of logarithms:
Therefore, half-life
can be calculated using the equation:
This equation shows that half-life
and the radioactive decay rate constant λ are inversely proportional
Therefore, the shorter the half-life, the larger the decay constant and the faster the decay
Worked Example
Strontium-90 is a radioactive isotope with a half-life of 28.0 years. A sample of Strontium-90 has an activity of 6.4 × 109 Bq.
Calculate the decay constant , in s–1, of Strontium-90.
Answer:
Step 1: Convert the half-life into seconds
= 28 years = 28 × (365 × 24 × 60 × 60) = 8.83 × 108 s
Step 2: Write the equation for half-life
Step 3: Rearrange for λ and calculate
Examiner Tips and Tricks
Although you may not be expected to derive the half-life equation, make sure you're comfortable with how to use it in calculations such as that in the worked example.
Half-Life from Decay Curves
The half-life of a radioactive substance can be determined from decay curves and log graphs
Since half-life is the time taken for the initial number of nuclei (or activity) to reduce by half, it can be found by
drawing a line to the curve at the point where the activity has dropped to half of its original value
drawing a line from the curve to the time axis, this is the half-life
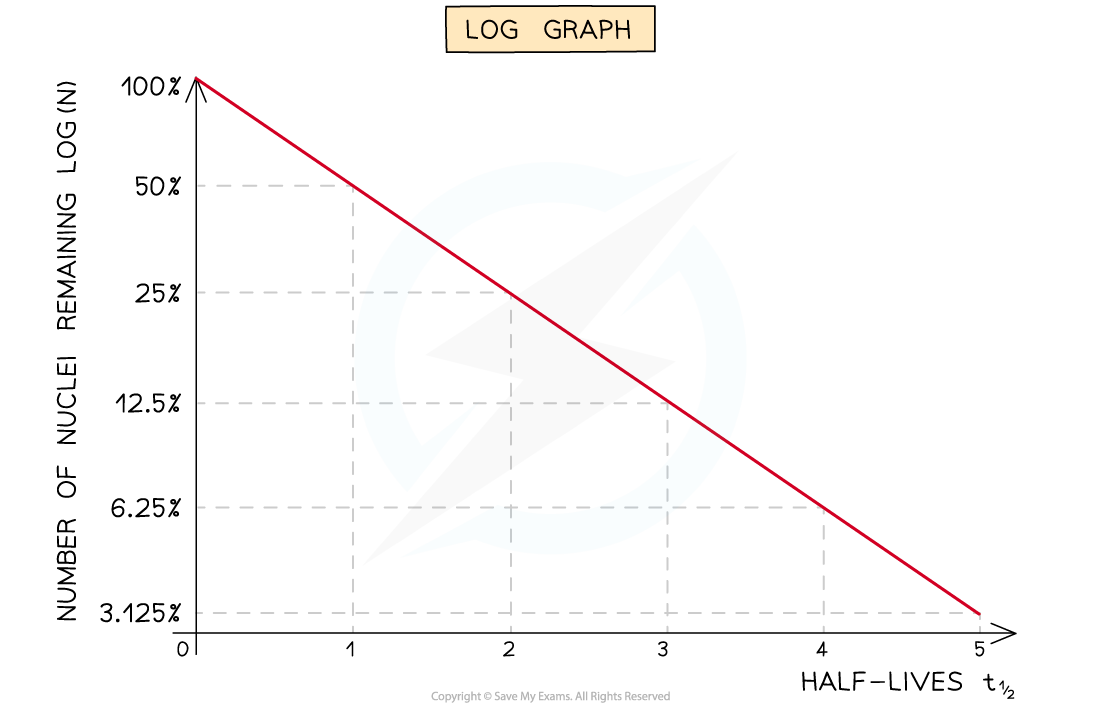
Log Graphs
Straight-line graphs tend to be more useful than curves for interpreting data
Nuclei decay exponentially, therefore, to achieve a straight line plot, logarithms can be used
Take the exponential decay equation for the number of nuclei
Taking the natural logs of both sides
In this form, this equation can be compared to the equation of a straight line
Where:
y-axis variable,
x-axis variable,
gradient,
y-intercept,
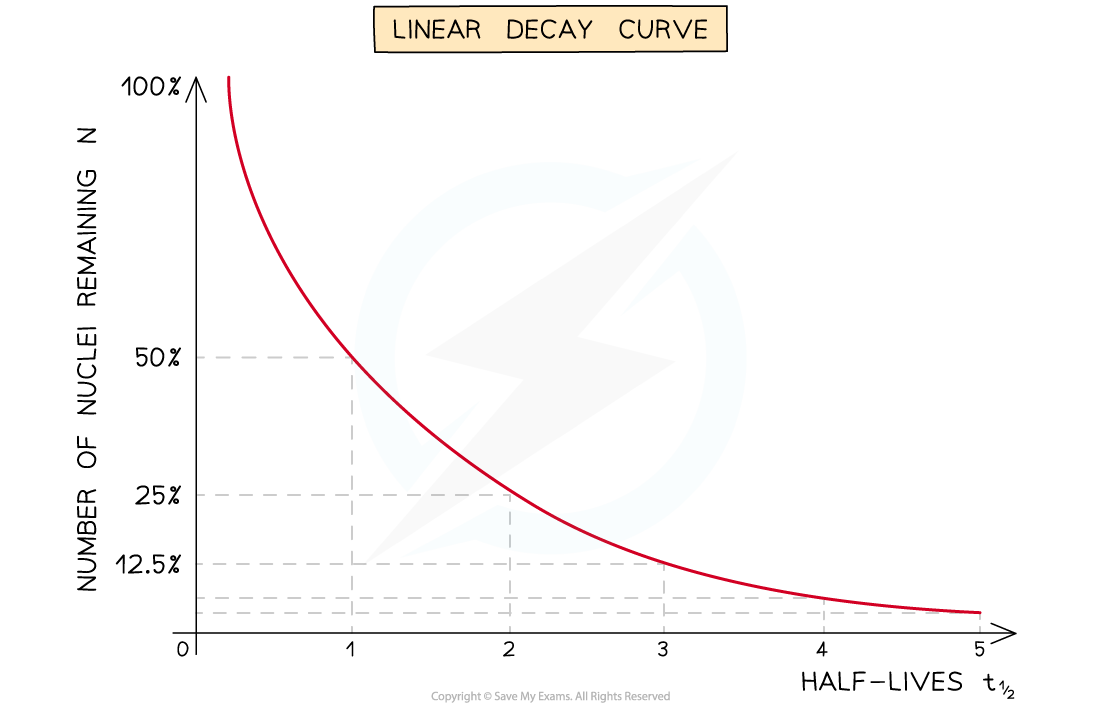
Half-lives can be found in a similar way to the decay curve but the intervals will be regular as shown below:
Worked Example
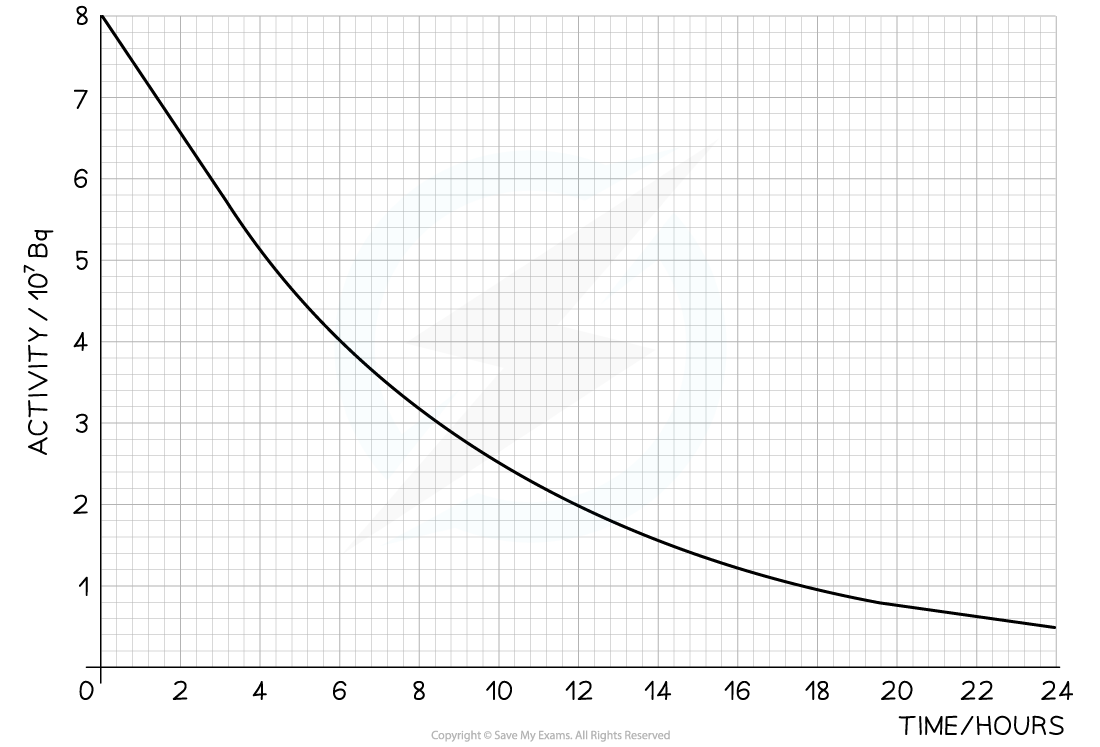
The radioisotope technetium is used extensively in medicine. The graph below shows how the activity of a sample varies with time.
Determine:
a) The decay constant for technetium
b) The number of technetium atoms remaining in the sample after 24 hours
Answer:
Part (a)
Step 1: Draw lines on the graph to determine the time it takes for technetium to drop to half of its original activity
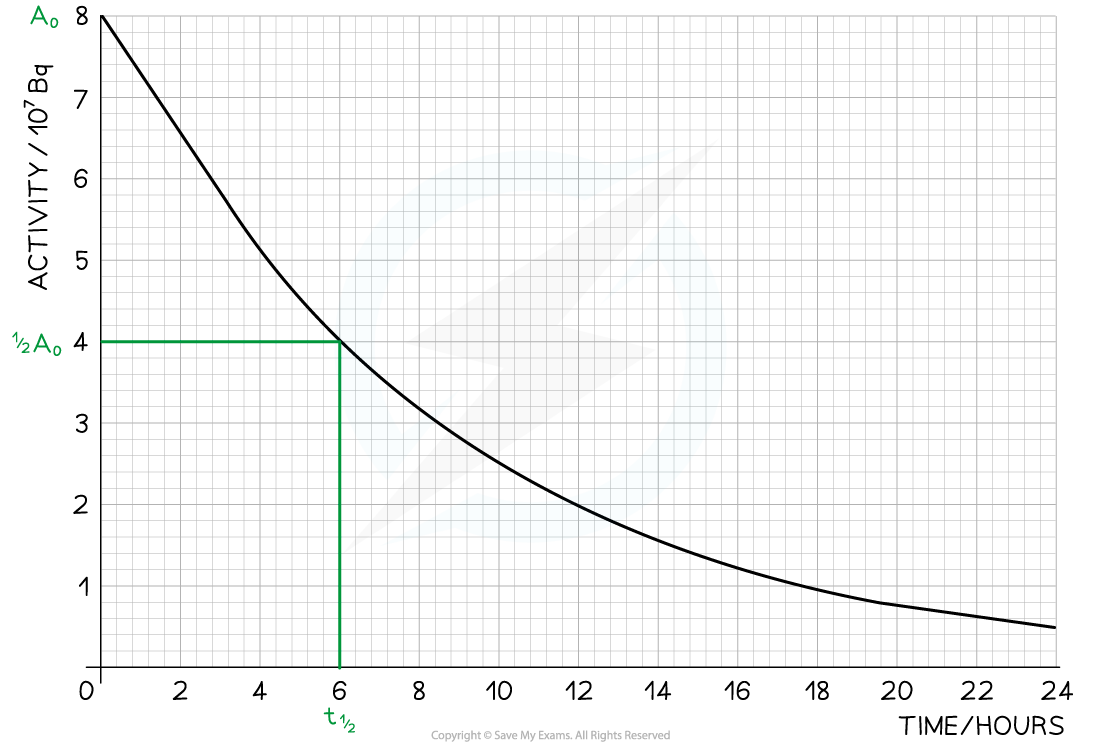
Step 2: Read the half-life from the graph and convert to seconds
= 6 hours = 6 × 60 × 60 = 21 600 s
Step 3: Write out the half life equation
Step 4: Calculate the decay constant
Part (b)
Step 1: Draw lines on the graph to determine the activity after 24 hours
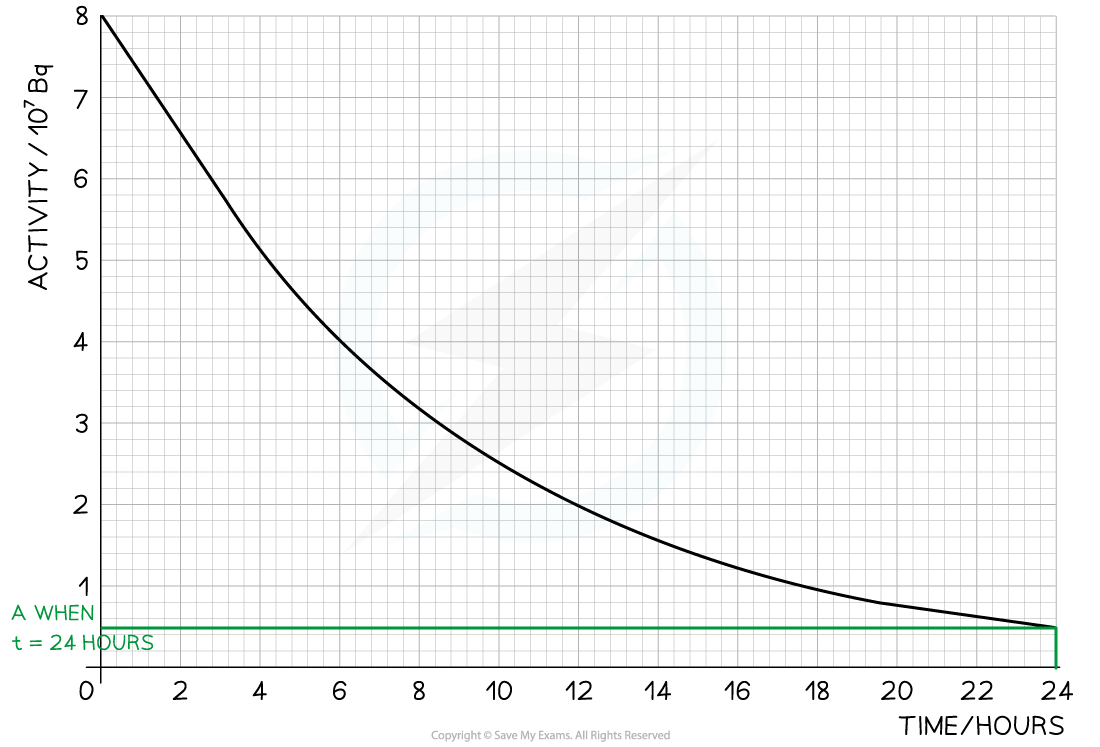
At
= 24 hours,
= 0.5 × 107 Bq
Step 2: Write out the activity equation
Step 3: Calculate the number of atoms remaining in the sample

Unlock more, it's free!
Did this page help you?