Exponential Decay (AQA A Level Physics): Revision Note
Exam code: 7408
Exponential Decay
In radioactive decay, the number of undecayed nuclei falls very rapidly, without ever reaching zero
Such a model is known as exponential decay
The graph of number of undecayed nuclei against time has a very distinctive shape:
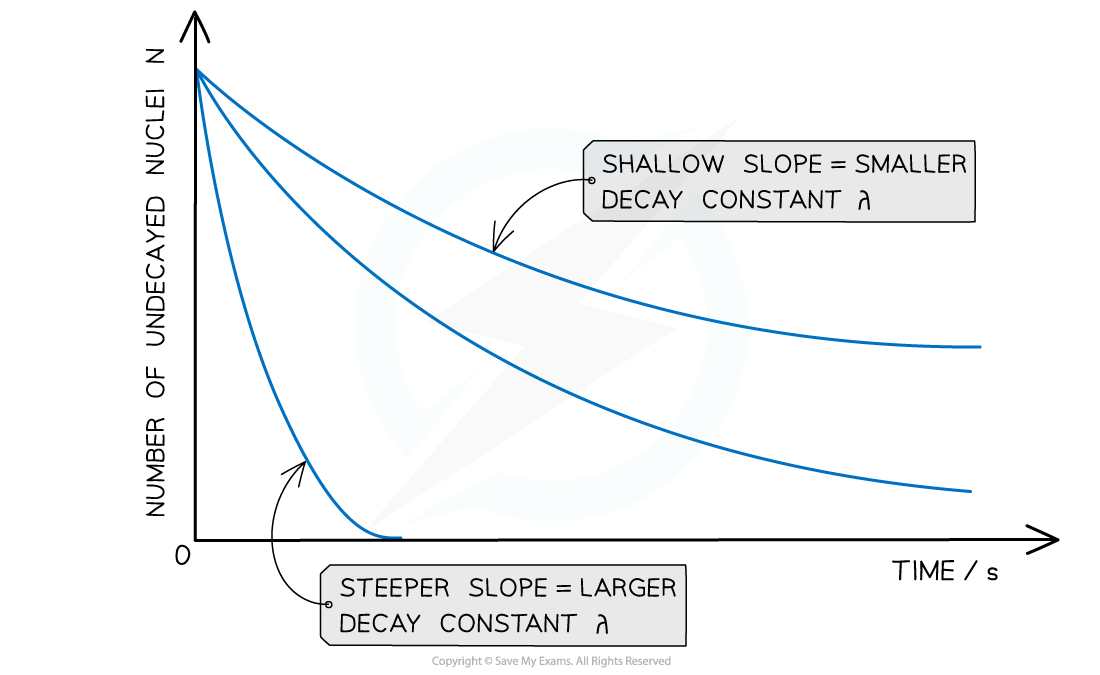
Radioactive decay follows an exponential pattern. The graph shows three different isotopes each with a different rate of decay
The key features of this graph are:
The steeper the slope, the larger the decay constant λ (and vice versa)
The decay curves always start on the y-axis at the initial number of undecayed nuclei (N0)
Equations for Radioactive Decay
The number of undecayed nuclei N can be represented in exponential form by the equation:
Where:
N0 = the initial number of undecayed nuclei (when t = 0)
N = number of undecayed nuclei at a certain time t
λ = decay constant (s-1)
t = time interval (s)
The number of nuclei can be substituted for other quantities.
For example, the activity A is directly proportional to N, so it can also be represented in exponential form by the equation:
Where:
A = activity at a certain time t (Bq)
A0 = initial activity (Bq)
The received count rate C is related to the activity of the sample, hence it can also be represented in exponential form by the equation:
Where:
C = count rate at a certain time t (counts per minute or cpm)
C0 = initial count rate (counts per minute or cpm)
The exponential function e
The symbol
represents the exponential constant
It is approximately equal to
= 2.718
On a calculator it is shown by the button
The inverse function of
is
, known as the natural logarithmic function
This is because, if
, then
Worked Example
Strontium-90 decays with the emission of a β-particle to form yttrium-90.
The decay constant of strontium-90 is 0.025 year -1.
Determine the activity of the sample after 5.0 years, expressing the answer as a fraction of the initial activity
.
Answer:
Step 1: Write out the known quantities
Decay constant, λ = 0.025 year -1
Time interval, t = 5.0 years
Both quantities have the same unit, so there is no need for conversion
Step 2: Write the equation for activity in exponential form
Step 3: Rearrange the equation for the ratio between A and A0
Step 4: Calculate the ratio A/A0
Therefore, the activity of strontium-90 decreases by a factor of 0.88, or 12%, after 5 years
Using Molar Mass & The Avogadro Constant
Molar mass
The molar mass, or molecular mass, of a substance is the mass of a substance, in grams, in one mole
Its unit is g mol-1
The number of moles from this can be calculated using the equation:
Avogadro’s constant
Avogadro’s constant
is defined as:
The number of atoms in one mole of a substance; equal to 6.02 × 1023 mol-1
For example, 1 mole of sodium (Na) contains 6.02 × 1023 atoms of sodium
The number of atoms, or nuclei, can be determined using the equation:
Worked Example
Americium-241 is an artificially produced radioactive element that emits α-particles.
In a smoke detector, a sample of americium-241 of mass 5.1 µg is found to have an activity of 5.9 × 105 Bq. The supplier’s website says the americium-241 in their smoke detectors initially has an activity level of 6.1 × 105 Bq.
Determine:
(a) the number of nuclei in the sample of americium-241
(b) the decay constant of americium-241
(c) the age of the smoke detector in years.
Answer:
Part (a)
Step 1: Write down the known quantities
Mass = 5.1 μg = 5.1 × 10−6 g
Molecular mass of americium = 241
Avogadro constant,
= 6.02 × 1023 mol−1
Step 2: Write down the equation relating number of nuclei, mass and molecular mass
Step 3: Calculate the number of nuclei
Number of nuclei: = 1.27 × 1016
Part (b)
Step 1: Write down the known quantities
Activity, A = 5.9 × 105 Bq
Number of nuclei, N = 1.27 × 1016
Step 2: Write the equation for activity
Activity:
Step 3: Rearrange for decay constant λ and calculate the answer
Part (c)
Step 1: Write down the known quantities
Activity, A = 5.9 × 105 Bq
Initial activity, A0 = 6.1 × 105 Bq
Decay constant, λ = 4.65 × 10–11 s–1
Step 2: Write the equation for activity in exponential form
Step 3: Rearrange for time t
Step 4: Calculate the age of the smoke detector and convert to years
s
= 22.7 years
Therefore, the smoke detector is 22.7 years old

Unlock more, it's free!
Did this page help you?