Alpha, Beta & Gamma Radiation (AQA A Level Physics): Revision Note
Exam code: 7408
Alpha, Beta & Gamma Radiation
Some isotopes of elements are unstable
This is usually due to an imbalance of protons and neutrons in a nucleus
To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay
The three main types of radioactive particle or radiation are:
Alpha particles
Beta particles
Gamma radiation
Alpha Particles
An alpha (α) particle is a high-energy helium nucleus
It contains 2 protons and 2 neutrons
It has a mass of 4u and a charge of +2e
Alpha particles are usually emitted from nuclei that are too large
The nuclear notation for an alpha particle is:

Nuclear notation for an alpha particle (a helium nucleus)
Alpha particles are the most ionising type of radiation
This is due to having the highest charge of +2e
They produce the highest number of ion pairs per cm in air (~10 000 ion pairs per cm)
This means they can do more damage to cells than the other types of radiation
Alpha particles are the least penetrating type of radiation
This means they travel the shortest distance in air before being absorbed
They have a range of around 3 to 7 cm in air
Beta Particles
A beta-minus (β−) particle is a high-energy electron
They are emitted by nuclei that have too many neutrons
A beta-plus (β+) particle is a high-energy positron (antimatter of electrons)
They are emitted by nuclei that have too many protons
The nuclear notation for beta-minus and beta-plus particles are:

Nuclear notation for beta minus and beta plus particle
Beta particles are a moderately ionising type of radiation
This is due to having a charge of ±1e
They produce a moderate number of ion pairs per cm in air (~100 ion pairs per cm)
This means they can do some slight damage to cells (less than alpha but more than gamma)
Beta particles are a moderately penetrating type of radiation
They have a range of around 20 cm to 3 m in air, depending on their energy
Gamma Radiation
Gamma (γ) rays are a type of high-energy electromagnetic radiation
They are emitted by nuclei that need to lose some energy
The nuclear notation for gamma radiation is:

Nuclear notation for gamma rays
Gamma is the least ionising type of radiation
This is because it is electromagnetic radiation (photon), which has no charge
Gamma produces the lowest number of ion pairs per cm in air (~1 ion-pair per cm)
It can still cause damage to cells, but not as much as alpha or beta radiation. This is why it is widely used for cancer radiotherapy
Gamma is the most penetrating type of radiation
This means it travels the furthest distance in air before being absorbed
Gamma radiation has an infinite range and follows an inverse square law
Comparing Alpha, Beta & Gamma
The properties of the different types of radiation are summarised in the table below:
Comparison of alpha, beta and gamma radiation

Ionising ability
If any type of radiation collides with an atom, it can knock out electrons, ionising the atom
This can cause chemical changes in materials and damage to living cells
The ionising ability of radiation can be quantified by the number of ion pairs it produces per cm of air
Alpha particles are the most ionising type of radiation, while gamma radiation is the least ionising type of radiation
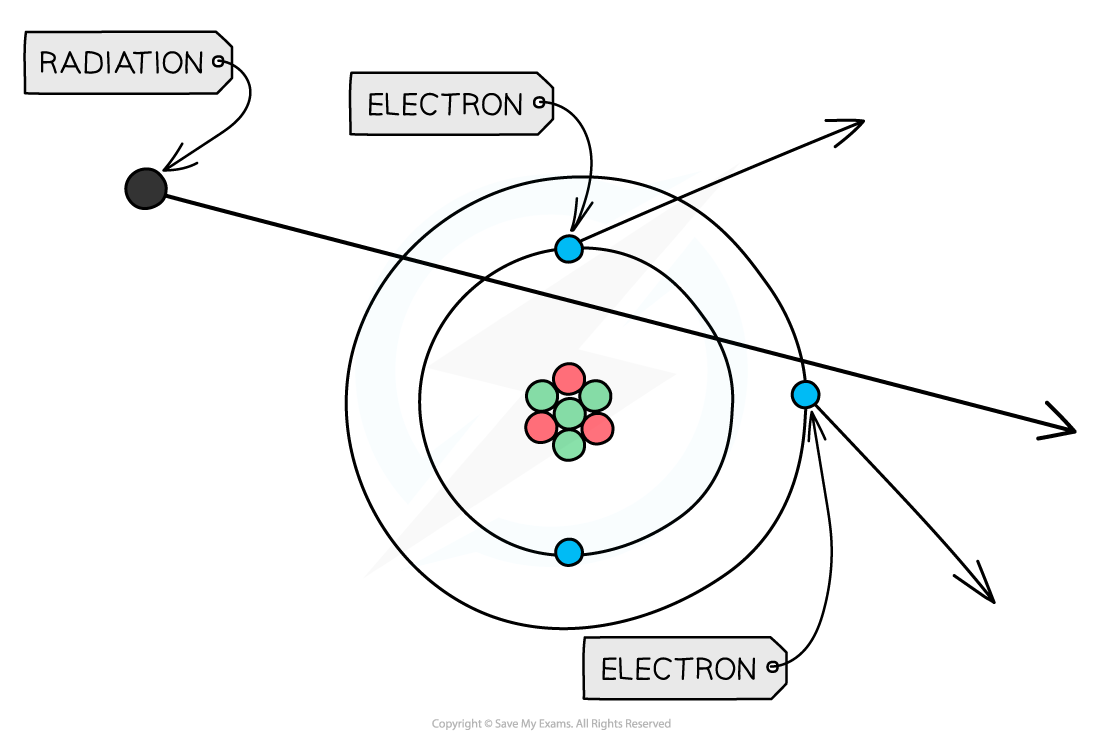
When radiation passes close to atoms, it can knock out electrons, ionising the atom
Penetrating power
The distance radiation can travel before being absorbed is described by its penetrating power
Alpha particles are the least penetrating, whereas gamma radiation is the most penetrating
Alpha particles can be stopped by a single sheet of paper
Beta particles can be stopped by a few millimetres of aluminium foil
The intensity of gamma radiation can be reduced by several metres of concrete or several centimetres of lead

Alpha particles are highly ionising and easily absorbed by atoms whereas gamma radiation is highly penetrating and requires very thick lead to reduce its intensity
Deflection in Electric and Magnetic Fields
When a charged particle enters an electric field it will undergo a deflection
Alpha particles are deflected towards the negative plate
Beta particles are deflected towards the positive plate
Gamma radiation is not deflected and travels straight through between the plates

Alpha and beta particles are deflected by an electric field
When a charged particle moves in a magnetic field, it will also undergo a deflection
Faster-moving particles move in larger circular paths according to the equation:
The larger the circular path, the greater the deflection
The amount of deflection of a particle depends on:
The speed of the particle, v
The mass of the particle, m
The charge on the particle, q
Alpha particles can be deflected slightly in strong electric and magnetic fields
Alpha particles have the highest charge, but also the greatest mass, so their high momentum means they deflect less than a beta particle (in a given field)
Beta particles can be deflected through large angles by electric and magnetic fields
Beta particles typically travel at much greater speeds than alpha particles, but have much less mass, so they deflect significantly more than an alpha particle (in a given field)
Gamma rays are not deflected in magnetic and electric fields as they are electrically neutral
However, they can transfer their energy to atomic electrons which can be deflected
Worked Example
Three successive radioactive decays are shown in the diagram below. Each one results in a particle being emitted.
The first decay results in the emission of a β-particle.
The second decay results in the emission of an α-particle.
The third decay results in the emission of another β-particle.

Nuclides W and Z are compared.
Which nuclide of Z is formed at the end of this decay?
A. B.
C.
D.
Answer: D
Step 1: Write the equation for the β− decay
A β− particle is an electron
The nucleon number stays the same
The proton number increases by 1
Step 2: Write the equation for the α decay
An α particle is a helium nucleus
The nucleon number reduces by 4
The proton number reduces by 2
Step 3: Write the equation for the β+ decay
A β+ particle is a positron
The nucleon number stays the same
The proton number reduces by 1
Step 4: Determine the final nucleon Z
The final nucleon, Z will be:
Applications of Alpha, Beta & Gamma
Smoke Detectors
Smoke detectors contain a small amount of Americium-241, which is a weak alpha source
Within the detector, alpha particles are emitted and cause the ionisation of nitrogen and oxygen molecules in the air
These ionised molecules enable the air to conduct electricity and hence a small current can flow
If smoke enters the alarm, it absorbs the alpha particles, hence reducing the current which causes the alarm to sound
Am-241 has a half-life of 460 years, meaning over the course of a lifetime, the activity of the source will not decrease significantly and it will not have to be replaced

The operation of a smoke detector
Thickness Controls
Beta radiation can be used to determine the thickness of aluminium foil, paper, plastic, and steel
The thickness can be controlled by measuring how much beta radiation passes through the material to a Geiger counter
Beta radiation must be used, because:
Alpha particles would be absorbed by all the materials
Gamma radiation would pass through undetected through the materials
The Geiger counter controls the pressure of the rollers to maintain the correct thickness
A source with a long half-life must be chosen so that it does not need to be replaced often

The pressure of the rollers can be adjusted to control the thickness of the aluminium foil depending on the amount of beta radiation detected
Worked Example
Below are listed four radioactive sources, together with the type of radiation they emit
A. Americium-241 Alpha (α)
B. Strontium-90 Beta Minus (β–)
C. Cobalt-60 Beta Minus (β–) & Gamma (γ)
D . Fluorine-18 Beta Plus (β+)
Which isotope is suitable for the purpose of:
a) Sterilising hospital equipment sealed inside plastic bags?
b) Discharging static electricity that has built up in the manufacture of polythene?
c) Monitoring the thickness of a thin metal being produced in a factory?
d) A smoke detector?
Part (a) Answer: C
Alpha and low energy beta radiation would most likely be absorbed by the bag
Therefore, gamma radiation, or very high energy beta particles, would be needed to penetrate the bag
This would be best suited to Cobalt-60
Part (b) Answer: D
Static electricity is an imbalance of electric charges on the surface of the polythene and is generally composed of negatively charged electrons
In order to get rid of the static charge, it will need to be neutralised
Beta-plus particles, or positrons, are the antimatter counterpart of the electron, and hence, are oppositely charged
When the positrons are directed at the surface of the polythene, the electrons will be attracted to them and become neutralised as the particles annihilate as they collide
Therefore, the beta-plus emitter, Fluorine-18, would be best suited to this job
Part (c) Answer: B
Alpha particles would not be suitable for measuring the thickness of metal as they can be stopped by a thin sheet of paper
Gamma rays are the most penetrating of the radiations and hence would not be suitable where thickness monitoring is up to a few millimetres as they would all pass through
Beta particles are ideally suited as they have enough energy to pass through thin sheets of metal and any changes in thickness would be easily detected
Therefore, the beta-minus emitter Strontium-90 would be the most suitable isotope
Part (d) Answer: A
Since smoke detectors are present inside homes and other buildings, they must pose no hazard to residents
This means the smoke detector must contain a very small amount of the radioactive material
Also, the radiation should not be too penetrating and should only be able to travel a few centimetres
Therefore, an alpha source should be selected – this means Americium-241 would be the most suitable isotope

Unlock more, it's free!
Did this page help you?