Motion of Charged Particles (AQA A Level Physics): Revision Note
Exam code: 7408
Motion of Charged Particles in an Electric Field
A charged particle in an electric field will experience a force on it that will cause it to move
If a charged particle remains stationary in a uniform electric field it will move parallel to the electric field lines (along or against the field lines depending on its charge)
If a charged particle is in motion through a uniform electric field (e.g. between two charged parallel plates), it will experience a constant electric force and travel in a parabolic trajectory
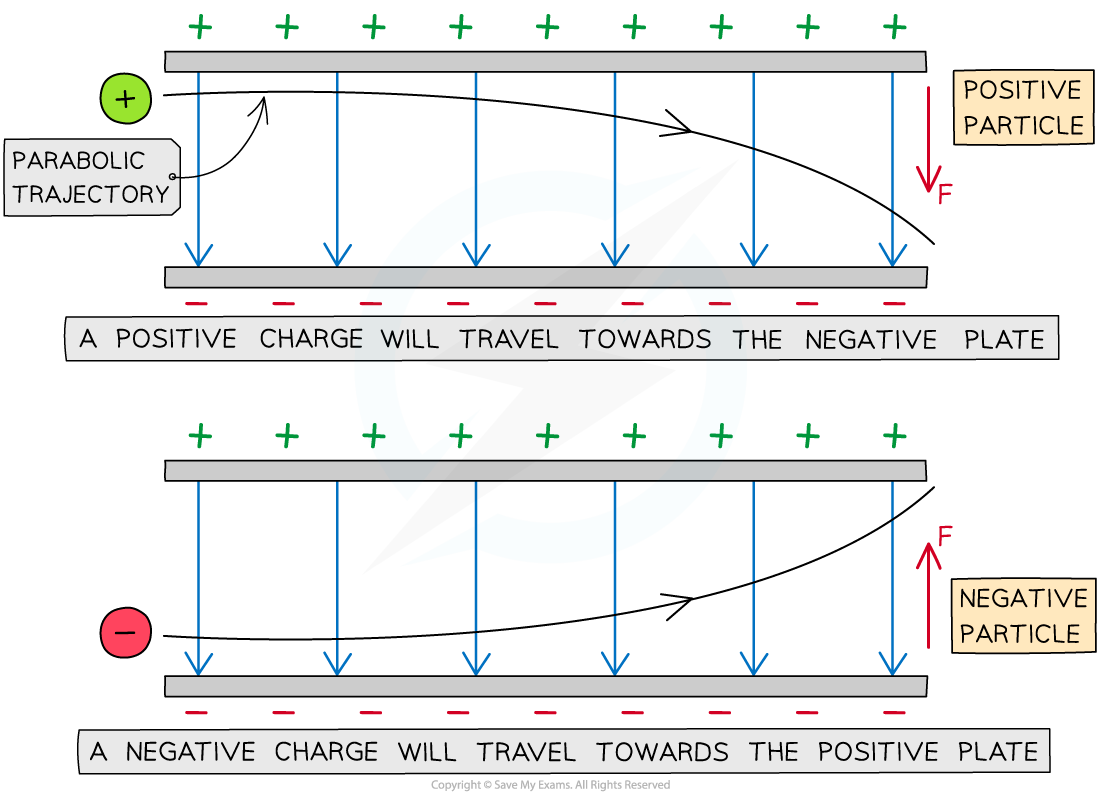
The parabolic path of charged particles in a uniform electric field
The direction of the parabola will depend on the charge of the particle
A positive charge will be deflected towards the negative plate
A negative charge will be deflected towards the positive plate
The force on the particle is the same at all points and is always in the same direction
Note: an uncharged particle, such as a neutron experiences no force in an electric field and will therefore travel straight through the plates undeflected
The amount of deflection depends on the following properties of the particles:
Mass – the greater the mass, the smaller the deflection and vice versa
Charge – the greater the magnitude of the charge of the particle, the greater the deflection and vice versa
Speed – the greater the speed of the particle, the smaller the deflection and vice versa
Worked Example
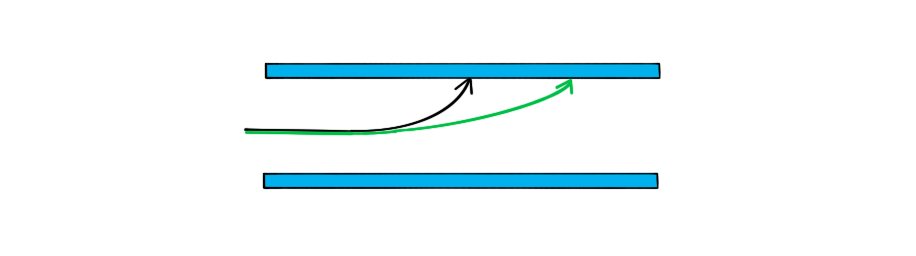
A single proton travelling with a constant horizontal velocity enters a uniform electric field between two parallel charged plates.
The diagram shows the path taken by the proton.

Draw the path taken by a boron nucleus that enters the electric field at the same point and with the same velocity as the proton.
Atomic number of boron = 5
Mass number of boron = 11
Answer:
Step 1: Compare the charge of the boron nucleus to the proton
Boron has 5 protons, meaning it has a charge 5 × greater than the proton
The force on boron will therefore be 5 × greater than on the proton
This is because electric force F is proportional to the charge Q
Step 2: Compare the mass of the boron nucleus to the proton
The boron nucleus has a mass of 11 nucleons meaning its mass is 11 × greater than the proton
The boron nucleus will therefore be less deflected than the proton
Step 3: Draw the trajectory of the boron nucleus
Since the mass comparison is much greater than the charge comparison, the boron nucleus will be much less deflected than the proton
The nucleus is positively charged since the neutrons in the nucleus have no charge
Therefore, the shape of the path will be the same as the proton
Examiner Tips and Tricks
Remember less deflection (e.g. from a higher mass particle) means the particle hits the plate later and the path has a smaller curve. Think of it as the particle being heavier so it is harder to steer it towards the plate.

Unlock more, it's free!
Did this page help you?