Gas Laws (AQA A Level Physics): Revision Note
Exam code: 7408
Ideal Gas Laws
The ideal gas laws are the experimental relationships between pressure (P), volume (V) and temperature (T) of an ideal gas
You need to be able to explain the following three ideal gas laws:
Boyles Law
Charles's Law
Pressure Law
The mass and the number of molecules of the gas is assumed to be constant for all of these
Boyle’s Law
If the temperature T of an ideal gas is constant, then Boyle’s Law is given by:

This means the pressure is inversely proportional to the volume of a gas
The relationship between the pressure and volume for a fixed mass of gas at constant temperature can also be written as:
P1V1 = P2V2
Where:
P1 = initial pressure (Pa)
P2 = final pressure (Pa)
V1 = initial volume (m3)
V2 = final volume (m3)
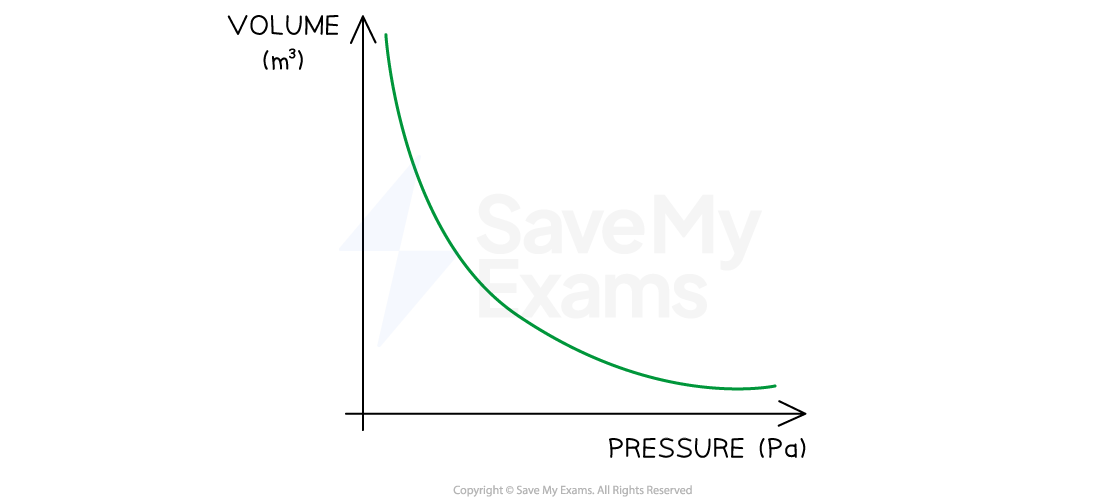
Boyle's Law graph representing pressure inversely proportional to volume
If the temperature is higher but still constant, then the graph has the same shape but is shifted further above the origin
Charles's Law
If the pressure P of an ideal gas is constant, then Charles’s law is given by:
V ∝ T
This means the volume is directly proportional to the temperature of a gas
The relationship between the volume and thermodynamic temperature for a fixed mass of gas at constant pressure can also be written as:

Where:
V1 = initial volume (m3)
V2 = final volume (m3)
T1 = initial temperature (K)
T2 = final temperature (K)
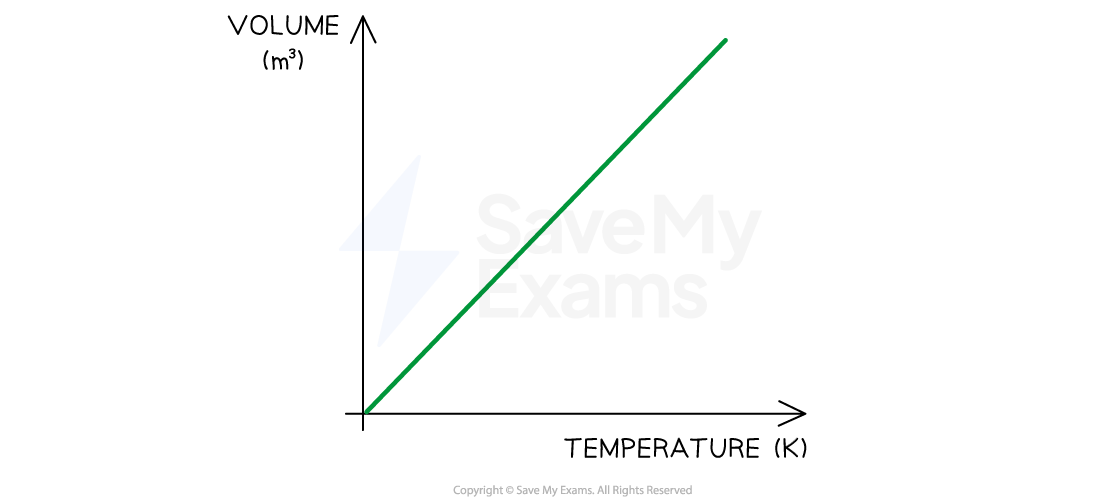
Charles's Law graph representing temperature (in K) directly proportional to the volume
A straight line through the origin shows direct proportionality
Pressure Law
If the volume V of an ideal gas is constant, the Pressure law is given by:
P ∝ T
This means the pressure is proportional to the temperature
The relationship between the pressure and thermodynamic temperature for a fixed mass of gas at constant volume can also be written as:

Where:
P1 = initial pressure (Pa)
P2 = final pressure (Pa)
T1 = initial temperature (K)
T2 = final temperature (K)

Pressure Law graph representing temperature (in K) directly proportional to the volume
A straight line through the origin shows direct proportionality between pressure and temperature in kelvin
Worked Example
The pressure inside a bicycle tyre is 5.10 × 105 Pa when the temperature is 279 K. After the bicycle has been ridden, the temperature of the air in the tyre is 299 K.
Calculate the new pressure in the tyre, assuming the volume is unchanged.
Answer:
Step 1: List the known quantities
Initial pressure,
Initial temperature,
Final temperature,
Step 2: Write out the relevant equation
Step 3: Rearrange to make the subject
Multiply both sides by
Step 4: Substitute in the known values to calculate
Step 5: Round to an appropriate amount of significant figures
The least precise input value is 3 s.f.
Therefore, the answer can only be given to this level of precision
Examiner Tips and Tricks
Remember when using any ideal gas law, including the ideal gas equation, the temperature T must always be in kelvin (K).
For Charles' Law, for example, if the x axis is temperature in degrees Celsius, not kelvin, the straight line no longer passes through the origin. 0 K is −273 °C so the line passes through the x axis at this value:

Relationships Between Pressure, Volume & Temperature
An ideal gas is one that obeys the relation:
pV ∝ T
Where:
p = pressure of the gas (Pa)
V = volume of the gas (m3)
T = thermodynamic temperature (K)
The molecules in a gas move around randomly at high speeds, colliding with surfaces and exerting pressure upon them
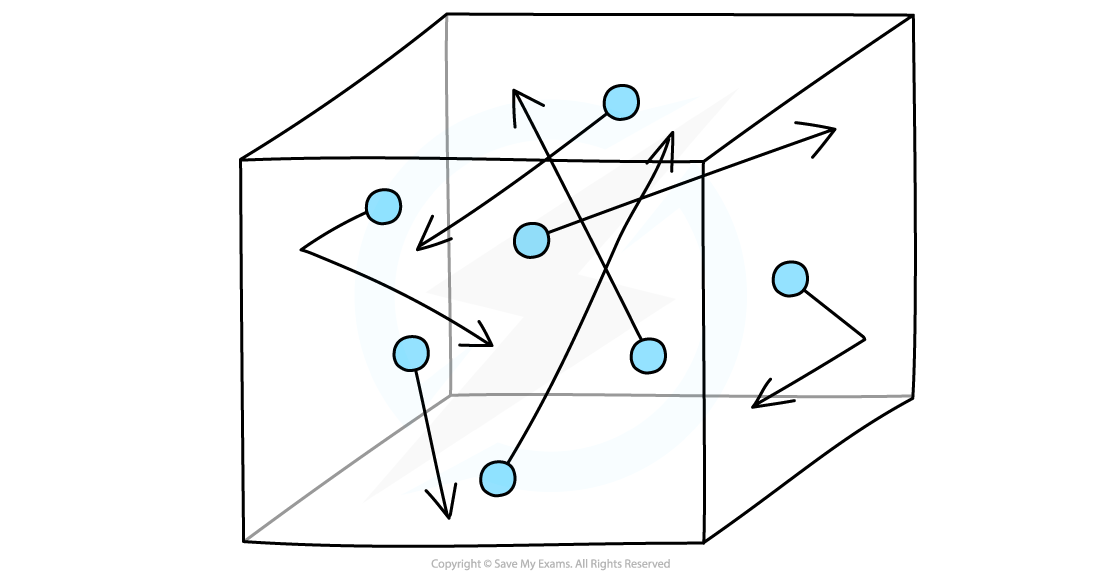
Gas molecules move about randomly at high speeds
Imagine molecules of gas free to move around in a box
The temperature of a gas is related to the average speed of the molecules:
The hotter the gas, the faster the molecules move
Hence the molecules collide with the surface of the walls more frequently
Since force is the rate of change of momentum:
Each collision applies a force across the surface area of the walls
The faster the molecules hit the walls, the greater the force on them
Since pressure is the force per unit area
Higher temperature leads to higher pressure
If the volume V of the box decreases, and the temperature T stays constant:
There will be a smaller surface area of the walls and hence more collisions
This also creates more pressure
Since this equates to a greater force per unit area, pressure in an ideal gas is therefore defined by:
The frequency of collisions of the gas molecules per unit area of a container
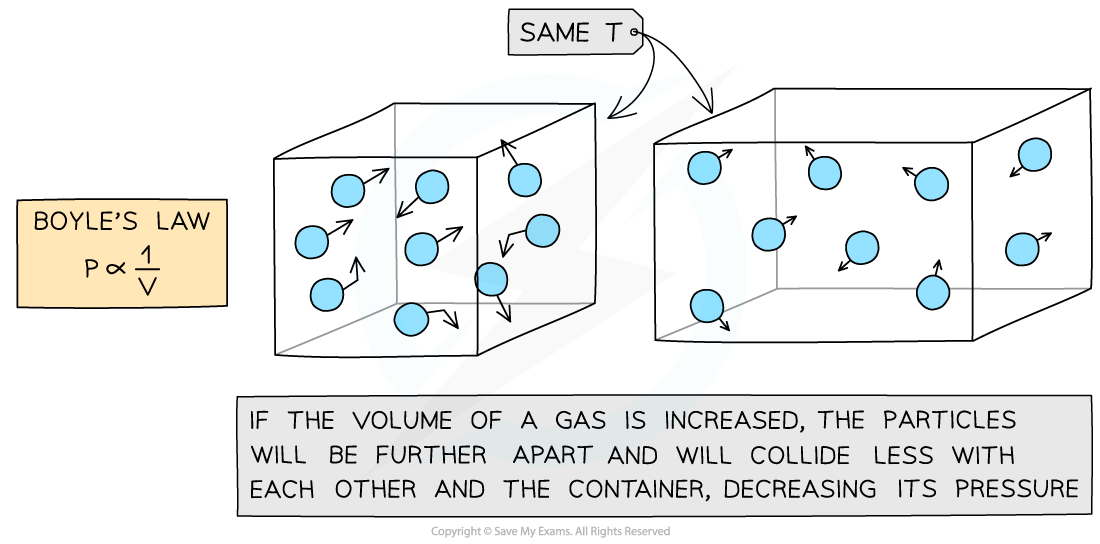
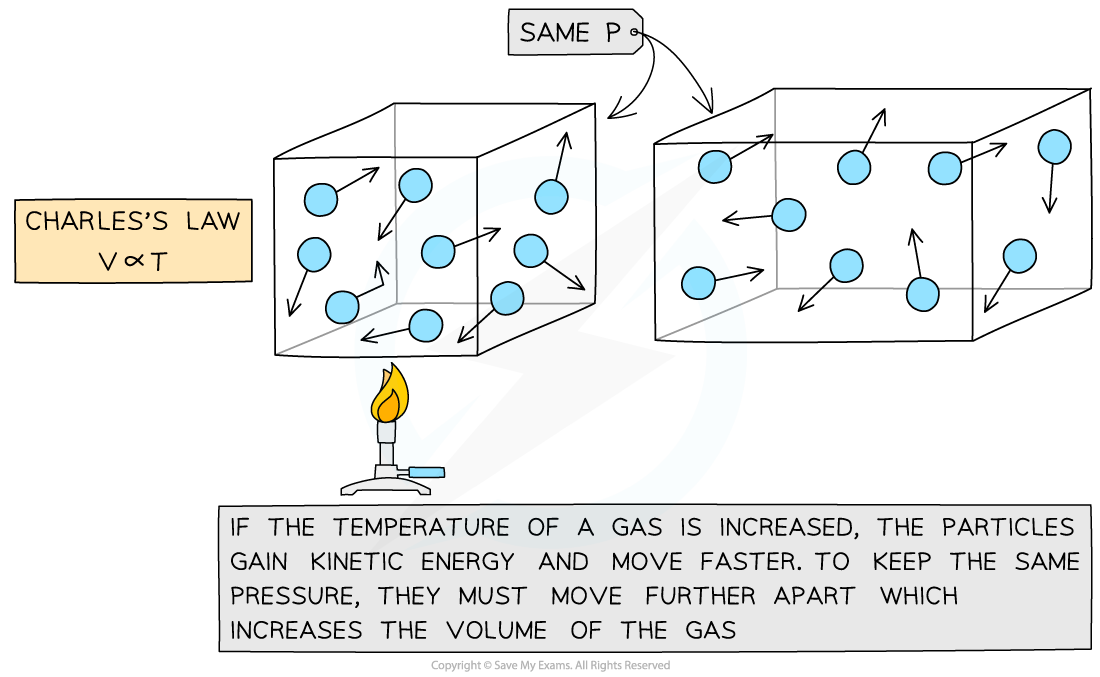
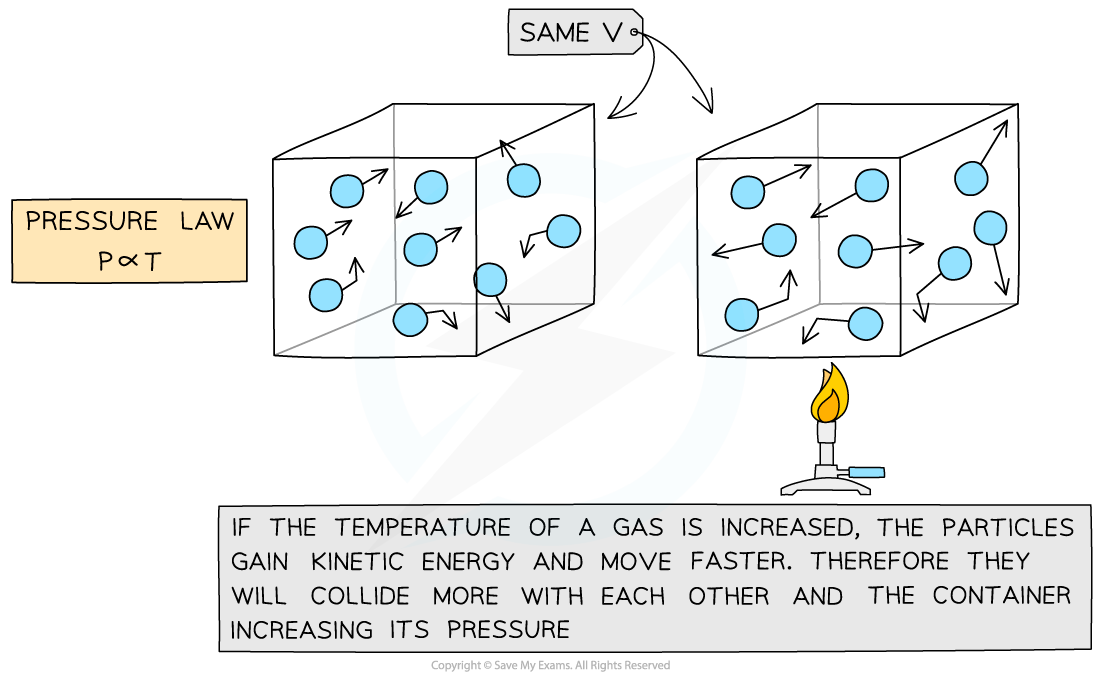
Molecular model of the three ideal gas laws
Worked Example
An ideal gas is in a container of volume 4.5 × 10-3 m3.The gas is at a temperature of 30 °C and a pressure of 6.2 × 105 Pa. Calculate the pressure of the ideal gas in the same container when it is heated to 40 °C.
Answer:
Step 1: Ideal gas relation between pressure, volume and temperature
pV ∝ T
Step 2: Write the equation in full
pV = kT
Where k = the constant of proportionality
Step 3: Rearrange for the constant of proportionality
Step 4: Convert temperature T into Kelvin
θ °C + 273.15 = T K
30 °C + 273.15 = 303.15 K
Step 5: Substitute in known value into constant of proportionality equation
Step 6: Rearrange ideal gas relation equation for pressure
Step 7: Substitute in new values
k = 9.203...
V stays the same = 4.5 × 10-3 m3
T = 40 °C = 40 + 273.15 = 313.15 K
Examiner Tips and Tricks
Don’t round too early in your working out! In the worked example, the unrounded value of k is represented by “…” to show its full value is to be carried over to the next step of the calculation. On your calculator, this can be done by using the “ans” button instead of typing in the whole number.

Unlock more, it's free!
Did this page help you?