Wave-Particle Duality (AQA A Level Physics) : Revision Note
Wave-Particle Duality
Light can behave as a particle (i.e. photons) and a wave
This phenomenon is called the wave-particle nature of light or wave-particle duality
Light interacts with matter, such as electrons, as a particle
The evidence for this is provided by the photoelectric effect
Light propagates through space as a wave
The evidence for this comes from the diffraction and interference of light in Young's Double-Slit Experiment
Diffraction occurs when waves pass through a gap or around an obstacle and the waves spread out

When light is passed through a slit with a width of similar size to the wavelength, the wave spreads out forming a diffraction pattern.
Light as a Particle
Einstein proposed that light can be described as a quanta of energy that behave as particles, called photons
The photon model of light explains that:
Electromagnetic waves carry energy in discrete packets called photons
The energy of the photons are quantised according to the equation E = hf
In the photoelectric effect, each electron can absorb only a single photon - this means only the frequencies of light above the threshold frequency will emit a photoelectron
The wave theory of light does not support the idea of a threshold frequency
The wave theory suggests any frequency of light can give rise to photoelectric emission if the exposure time is long enough
This is because the wave theory suggests the energy absorbed by each electron will increase gradually with each wave
Furthermore, the kinetic energy of the emitted electrons should increase with radiation intensity
However, in the photoelectric effect, this is not what is observed
If the frequency of the incident light is above the threshold and the intensity of the light is increased, more photoelectrons are emitted per second
Although the wave theory provides good explanations for phenomena such as interference and diffraction, it fails to explain the photoelectric effect
Compare wave theory and particulate nature of light

Electron Diffraction
Louis de Broglie discovered that matter can behave as a wave
Experiments show that a diffraction pattern is produced when a beam of electrons is directed at a thin graphite film
Diffraction is a property of waves, and cannot be explained by describing electrons as particles
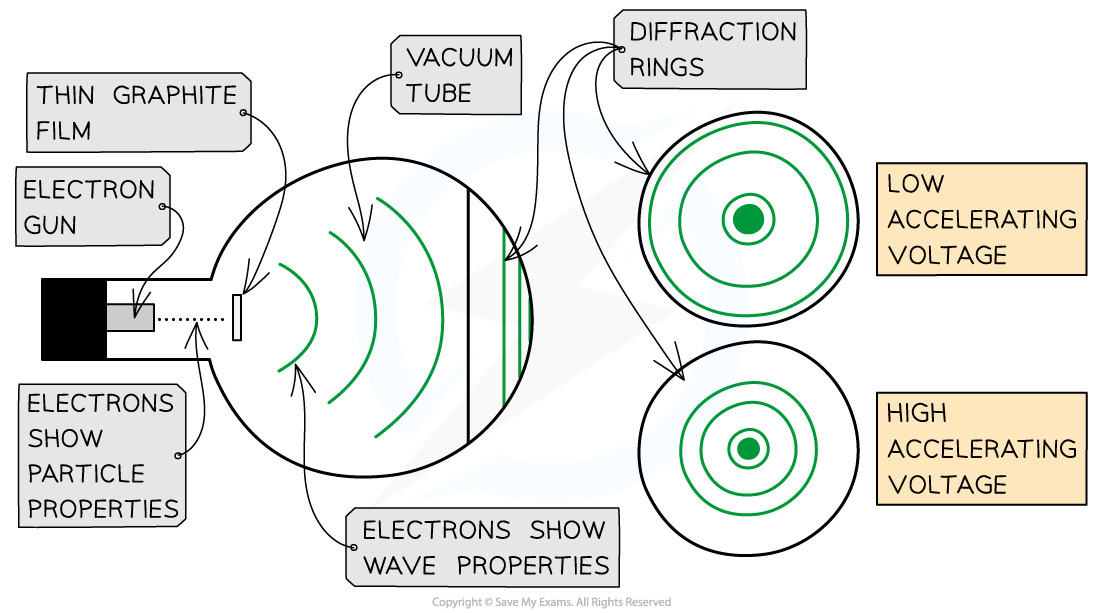
Electrons accelerated through a high potential difference demonstrate wave-particle duality
In order to observe the diffraction of electrons, they must be focused through a gap similar in size to their de Boglie wavelength, such as an atomic lattice
Graphite film is ideal for this purpose because of its crystalline structure
The gaps between neighbouring planes of the atoms in the crystals act as slits, allowing the electron waves to spread out and create a diffraction pattern
The diffraction pattern is observed on the screen as a series of concentric rings
This phenomenon is similar to the diffraction pattern produced when light passes through a diffraction grating
If the electrons acted as particles, a pattern would not be observed, instead, the particles would be distributed uniformly across the screen
It is observed that:
a larger accelerating voltage reduces the diameter of a given ring
a lower accelerating voltage increases the diameter of the rings
Investigating Electron Diffraction
Electron diffraction tubes can be used to investigate the wave properties of electrons
The electrons are accelerated in an electron gun to a high potential, such as 5000 V, and are then directed through a thin film of graphite
The electrons diffract from the gaps between carbon atoms and produce a circular pattern on a fluorescent screen made from phosphor
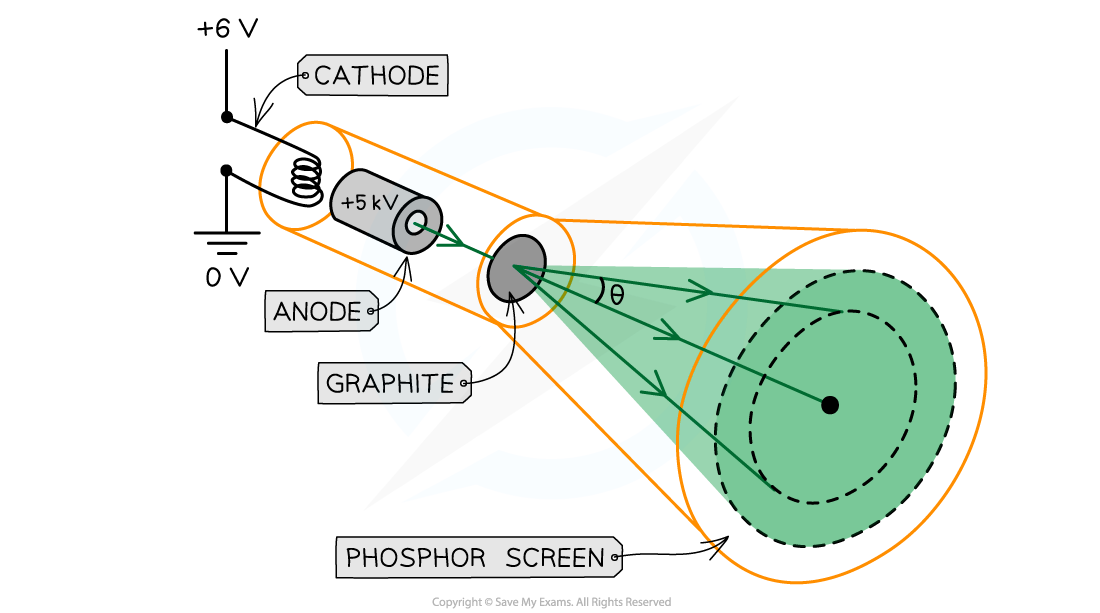
Experimental setup to demonstrate electron diffraction
Increasing the voltage between the anode and the cathode causes the energy, and hence speed, of the electrons to increase
The kinetic energy of the electrons is proportional to the voltage across the anode-cathode:
Ek = ½ mv2 = eV

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
