Alpha & Beta Decay (AQA A Level Physics) : Revision Note
α & β Decay Equations
When nuclei are unstable, they can become more stable through the process of radioactive decay
Three of the most common decay mechanisms are:
Alpha decay
Beta-minus decay
Beta-plus decay
Alpha Decay
Alpha decay is common in large, unstable nuclei with too many nucleons (protons and neutrons)
The decay involves a nucleus emitting an alpha particle and decaying into a different nucleus
An alpha particle consists of 2 protons and 2 neutrons
This is equivalent to a helium nucleus
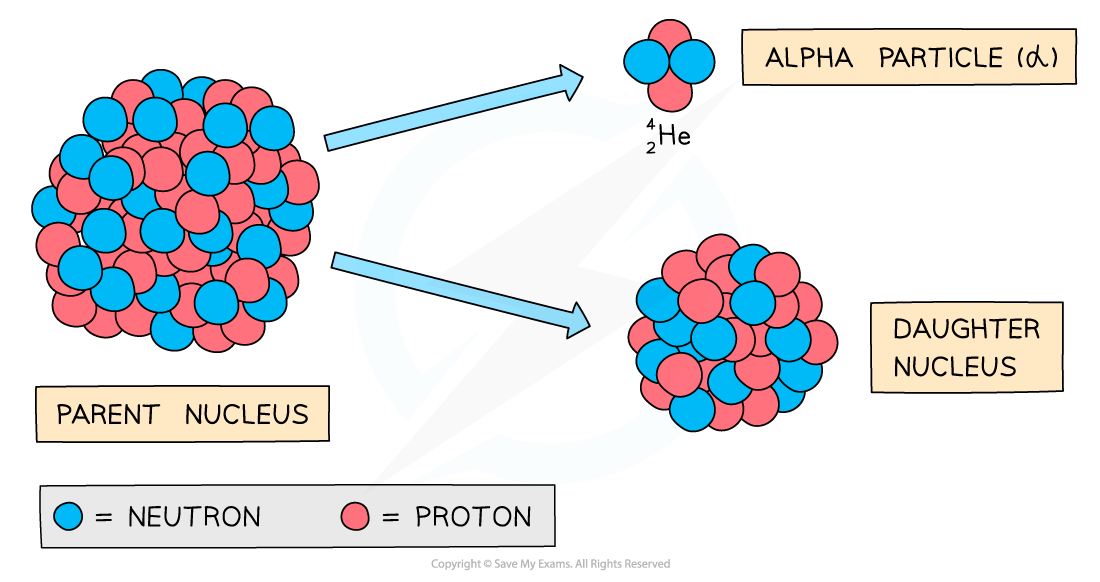
During alpha decay, a parent nucleus becomes a daughter nucleus by emitting an alpha particle (helium nucleus)
When an unstable nucleus (the parent nucleus) emits radiation, the constitution of its nucleus changes
As a result, the isotope will change into a different element (the daughter nucleus)
Alpha decay can be represented by the following radioactive decay equation:

When an alpha particle is emitted from a nucleus:
The nucleus loses 2 protons: proton number decreases by 2
The nucleus loses 4 nucleons: nucleon number decreases by 4
Beta-Minus Decay
A beta-minus, β-, particle is a high energy electron emitted from the nucleus
β- decay is when a neutron turns into a proton emitting an electron and an anti-electron neutrino
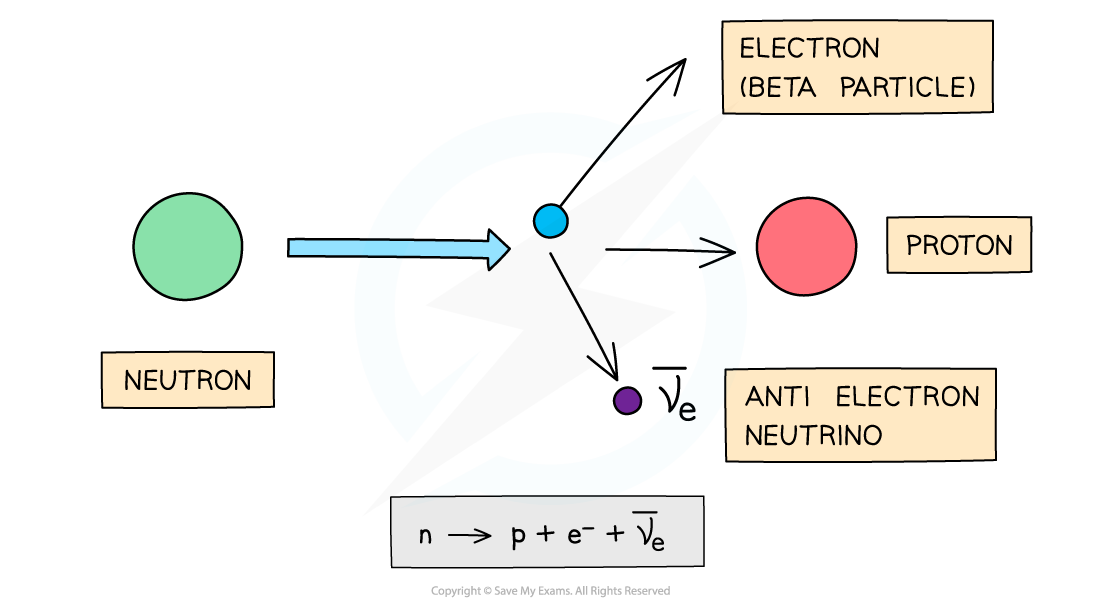
During beta-minus decay, a neutron in a parent nucleus becomes a proton in a daughter nucleus by emitting a beta-minus particle (an electron) and an anti-electron neutrino
When a β- particle is emitted from a nucleus:
The number of protons increases by 1: proton number increases by 1
The total number of nucleons stays the same: nucleon number remains the same

The new nucleus formed from the decay is called the “daughter” nucleus (nitrogen in the example above)
Beta-Plus Decay
A beta-plus, β+, particle is a high energy positron emitted from the nucleus
β+ decay is when a proton turns into a neutron emitting a positron (anti-electron) and an electron neutrino
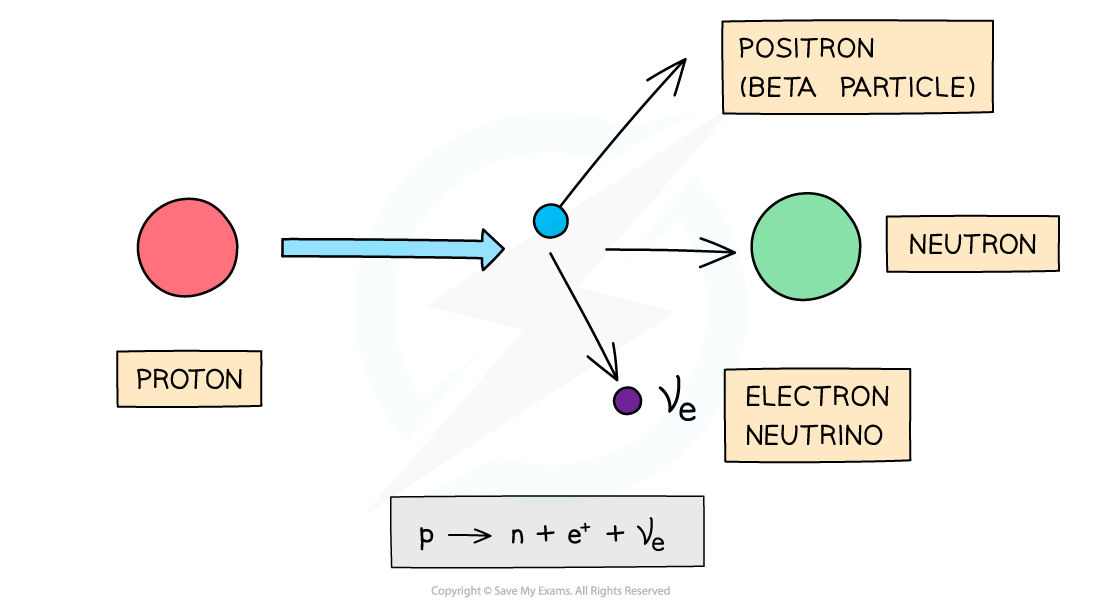
During beta-plus decay, a proton in a parent nucleus becomes a neutron in a daughter nucleus by emitting a beta-plus particle (a positron) and an electron neutrino
When a β+ particle is emitted from a nucleus:
The number of protons decreases by 1: proton number decreases by 1
The total number of nucleons stays the same: nucleon number remains the same

Worked Example
The radioactive nucleus undergoes alpha decay into a daughter nucleus Po.

(a) Which letter in the diagram represents the daughter product?
(b) What is the nucleon number and proton number of Po?
Answer:
Part (a)
Letter C represents the daughter product
The number of neutrons in
is 222 − 86 = 136
In alpha decay, the parent nucleus loses a helium nucleus (2 protons, 2 neutrons)
Proton number: 86 decreases to 84
Neutron number: 136 decreases to 134

Therefore, the correct answer is C
Part (b)
The equation for alpha decay is as follows:

Hence the daughter nucleus Po has
Nucleon number = 222 − 4 = 218
Proton number = 86 − 2 = 84
Worked Example
A radioactive substance with a nucleon number of 212 and a proton number of 82 decays by β-plus emission into a daughter product which further decays by β-plus emission into a granddaughter product.

Which letter in the diagram represents the granddaughter product?
Answer: A
The number of neutrons in the parent nucleus is 212 − 82 = 130
In beta-plus decay, a proton turns into a neutron
Proton number: 82 decreases to 80
Neutron number: 130 increases to 132

Therefore, the correct answer is A
Examiner Tips and Tricks
Remember to avoid the common mistake of confusing the number of neutrons with the nucleon number. In alpha decay, the nucleon (protons and neutrons) number decreases by 4 but the number of neutrons only decreases by 2.
Neutrino Emission
An electron neutrino is a type of subatomic particle with no charge and negligible mass which is also emitted from the nucleus
The anti-neutrino is the antiparticle of a neutrino
Electron anti-neutrinos are produced during β– decay
Electron neutrinos are produced during β+ decay

Although the neutrino has no charge and negligible mass, its existence was hypothesised to account for the conservation of energy in beta decay
When the number of α particles is plotted against kinetic energy, there are clear spikes that appear on the graph
This demonstrates that α-particles have discrete energies (only certain values)

Alpha particles have discrete energy levels whilst beta particles have a continuous range of energies
When the number of β particles is plotted against kinetic energy, the graph shows a curve
This demonstrates that beta particles (electrons or positrons) have a continuous range of energies
This is because the energy released in beta decay is shared between the beta particles (electrons or positrons) and neutrinos (or anti-neutrinos)
This was one of the first clues of the neutrino’s existence
The principle of conservation of momentum and energy applies in both alpha and beta emission
Examiner Tips and Tricks
One way to remember which particle decays into which depends on the type of beta emission, think of beta ‘plus’ as the ‘proton’ that turns into the neutron (plus an electron neutrino)

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
