Bertozzi's Experiment (AQA A Level Physics) : Revision Note
Bertozzi's Experiment
How do you Measure Kinetic Energy?
When accelerating a particle with charge q from rest, the work done by an electric field with potential difference V is equal to the energy gained by the particle's kinetic store, Ek :
This gives a way of measuring the kinetic energy of a particle of known charge, such as an electron
What was Bertozzi's Experiment?
Bertozzi accelerated electrons to speeds close to the speed of light and measured their kinetic energies
The aim of the experiment was to plot the squared speeds of electrons against their kinetic energies and compare the results to Newtonian and relativistic predictions
A strong electric field was used to transfer energy to the kinetic store of the electrons
This meant that, for an electric field of potential difference V, each electron had a kinetic energy of eV, where e is the magnitude of charge on an electron
These electrons were fired at an aluminium target
The speed of the electrons was simply calculated using distance divided by time
Once the electrons left the electric field, they travelled at constant speed, as no forces acted
A signal after the electric field and a signal attached to the aluminium target were used to measure a time interval using an oscilloscope
The distance was measured between the two signals and speed was calculated
A diagram outlining the experimental setup of Bertozzi's electron experiment
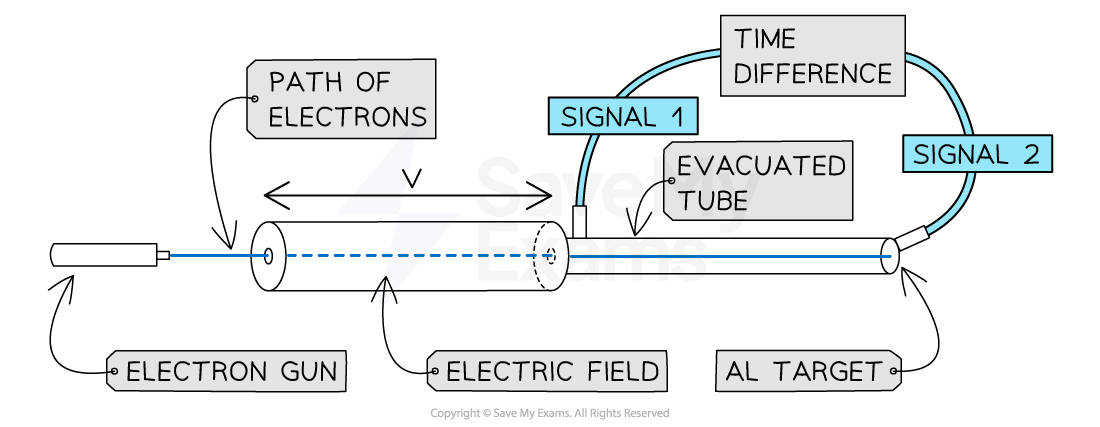
Electrons are accelerated by an electric field and trigger a signal once they have left the field and trigger another when they hit the aluminium target, allowing speed to be calculated
The Results
With several values of speed-squared and kinetic energy, Bertozzi produced a graph showing the following results
A graph showing speed-squared against kinetic energies
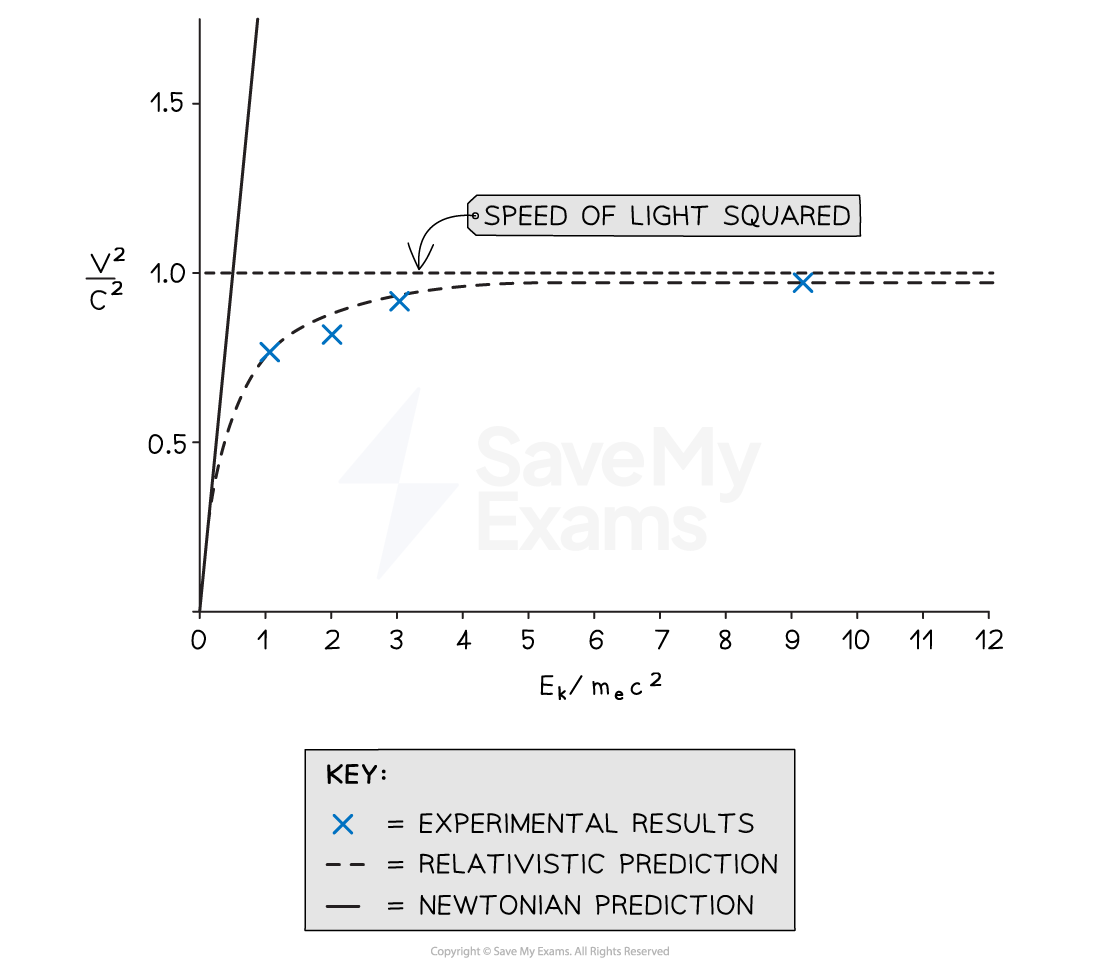
This graph shows the kinetic energy of the electrons, with units of mec2, plotted against their squared speeds, with units of c2. The Bertozzi results agree closely with the relativistic predictions of speeds and show electrons cannot exceed the speed of light.
This experiment's data agreed with the relativistic predictions far more than the Newtonian predictions
Verifying the Kinetic Energy of the Electrons
To avoid any doubts about whether the electrons actually did have a kinetic energy equal to eV, Bertozzi and his team measured the kinetic energy of the electrons directly as they hit the aluminium target
They did this by measuring the temperature change of the target and using the equation ΔE = mcΔθ
This energy change is equal to the total kinetic energy lost by the incoming electrons
The total charge transferred to the target was used to calculate the number of electrons which hit the target
With total energy transferred to the target and the number of electrons known, the team could calculate the kinetic energy per electron
These values were in agreement with the energy transferred to the electrons by the electric field, showing the results were valid and the electrons behaved relativistically at speeds close to c
Worked Example
In a repeat of the Bertozzi experiment, electrons are fired through an evacuated tube at a 0.0250 kg aluminium plate. After the experiment has run for a time, the target has accumulated −6.20 μC of charge and has increased in temperature by 1.80 °C.
Calculate the kinetic energy per electron in units of MeV.
Specific heat capacity of aluminium = 902 J kg-1 K-1
Answer:
Step 1: List the known quantities
Mass of aluminium, m = 0.0250 kg
Specific heat capacity of aluminium, c = 902 J kg-1 K-1
Change in temperature, Δθ = 1.80 °C
Charge transferred to aluminium plate, Q = −6.20 μC
Charge of an electron, e = −1.60 × 10−19 C
Step 2: Write out the relevant equation
Thermal energy transferred to aluminium, ΔE = mcΔθ
Step 3: Calculate the energy transferred to the aluminium's thermal store
The thermal energy gained by the plate is:
Step 4: Calculate the number of electrons incident on the aluminium plate
Each electron transfers a charge of e to the plate
The total charge transferred to the plate divided by e will give the number of electrons, n, incident on the plate:
Step 5: Calculate the kinetic energy per electron
The total thermal energy gained by the plate is equal to the total kinetic energy, Ek,tot , of all the electrons hitting it
Ek,tot divided by the number of electrons gives the kinetic energy per electron, Ek :
Step 6: Convert the kinetic energy per electron into mega-electronvolts
Recall that 1 eV is equal to 1.60 × 10-19 J:
Finally, the answer must be to 3 significant figures, as all the figures in the question were given to this number:
Examiner Tips and Tricks
You may be asked on either method of determining the kinetic energy of each electron so expect to see the thermal energy equation and work done by an electric field in this topic.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
