The Discovery of Photoelectricity (AQA A Level Physics): Revision Note
Exam code: 7408
Classical Wave Theory & Photoelectricity
How did the Photoelectric Effect Contradict Wave Theory?
The details of the photoelectric effect have already been covered in the Particles & Radiation section of this course
In the photoelectric effect, incident radiation on a metal's surface causes it to emit electrons
However, this only happens for radiation above a certain frequency
If the radiation is below this threshold frequency, no matter how great the intensity, photoelectrons will not be emitted from the metal's surface
A diagram recapping the process of photoelectric emission
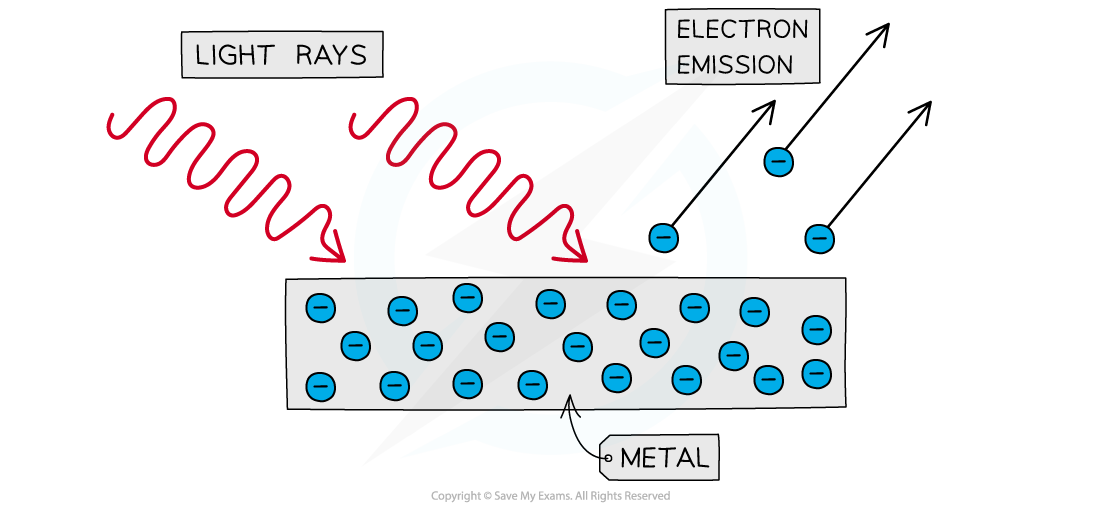
Light rays with a frequency above the metal's threshold frequency cause immediate emission of electrons from the surface
This was in direct contradiction with wave theory, which predicted that an electromagnetic wave transferred energy continuously
According to wave theory, if low frequency radiation was aimed at the metal at a high enough intensity, then enough energy would be transferred to remove photoelectrons
However, as soon as a radiation above the threshold frequency was shone on the metal's surface, even at low intensities, photoelectrons were immediately emitted
Additionally, the intensity of incident radiation only affected the number of photoelectrons emitted, but not the energy they left with
All of this evidence contradicted the idea that electromagnetic radiation transfers energy like a wave does
Explanation of Photoelectricity
Einstein's Explanation
In 1905 Einstein released three ground-breaking papers, one of which was a theoretical explanation of the photoelectric effect - this explanation built on Max Planck's work on black-bodies
Einstein proposed that electromagnetic radiation was made from discrete quanta, or packets, of energy of the size:
Where
h is Planck's constant and f is the frequency of the radiation
These quanta were called photons at a later date
These photons were massless in Einstein's theoretical description
This theory was able to explain the experimental results of the photoelectric effect
To explain why radiation below the threshold frequency didn't cause photoelectric emission:
Only one photon was able to transfer its energy to only one electron
If hf was not large enough to sufficiently energise an electron, the photons could not combine to energise that electron
To explain why the energy of emitted photoelectrons increased with the frequency of incident light:
Photons transferred all of their energy hf to electrons
If this was greater than the energy needed to emit the electrons, the rest of the energy was transferred to the kinetic store of the electrons
If hf was larger, more energy was left over for the kinetic store of the electrons
Once this was all experimentally confirmed 10 years later, Einstein received a Nobel Prize in Physics
Worked Example
Zinc has a threshold frequency of 6.5 × 1014 Hz.
Explain why wave theory predicted that photoelectrons would be emitted if zinc was exposed to electromagnetic radiation of 6.3 × 1014 Hz for long enough. Explain why this is not possible, using photon theory.
Answer:
Step 1: Recall the way waves transfer energy:
Wave theory predicted that electromagnetic radiation transferred energy continuously, as waves do
Over time, the electrons would be given enough energy to be removed from the metal
Step 2: Recall how energy is transferred in photon theory:
In photon theory, one photon transfers all of its energy to one electron
The photons of radiation with a frequency of 6.3 × 1014 Hz do not have enough energy to sufficiently energise the electrons

Unlock more, it's free!
Did this page help you?