Stokes' Law (AQA A Level Physics): Revision Note
Exam code: 7408
Motion of a Falling Oil Droplet
Motion of the Oil Droplet without the Electric Field
Millikan's experiment was designed to determine the charge of oil droplets, and therefore the charge on electrons
To calculate charge using the condition for stationary oil droplets, however, the mass of each oil droplet was required
With the electric field turned off, the oil droplets fell at terminal velocity
This means their weight, mg, was balanced by drag forces as they passed through the air
Millikan timed the oil drops as they passed the window of his apparatus to determine their speeds
A diagram showing weight and drag forces on an oil droplet
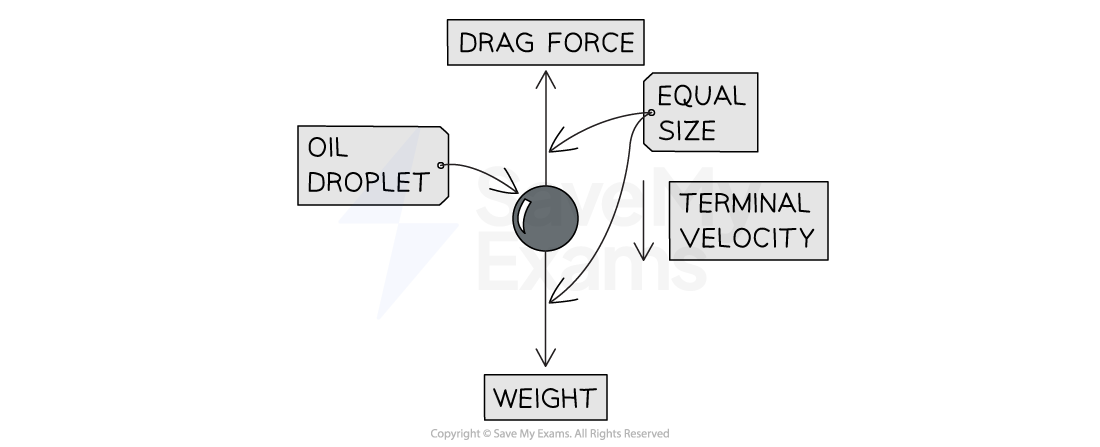
At terminal velocity, speed is constant and resultant force is zero, so weight is balanced by drag forces
Stokes' Law
Viscous Drag Force using Stokes' Law
In fluid dynamics, Stokes' law allows drag force on an object through a fluid (such as air) to be calculated, provided:
The object is small
The object is spherical
The speed is low
Stokes' law states that, for an object with radius r moving at velocity v through a fluid with viscosity η, the viscous drag force, F, is:
Calculating Mass from Stokes' Law
Recall that the drag force and weight are equal at terminal velocity, so:
Both mass and radius are unknown, but we can write mass in terms of radius for a sphere of known density, ρ :
Combining these equations allowed Millikan to determine the radius, and therefore mass, of an oil drop whose velocity was known
Recall the condition for a stationary oil drop:
This now gave Millikan enough information to calculate the charge Q on an oil drop if he knew its terminal velocity and the potential difference V required to make it stationary
Significance of Millikan's Results
Millikan repeated his experiment for many different oil droplets
They all had different charges, but he noticed an interesting pattern in his results
Every value of charge was an integer multiple of 1.60 × 10−19 C
Table of results from Millikan's Experiment
Oil Droplet Number | Charge on Droplet (× 10−19 C) | Charge divided by 1.60 × 10−19 C |
|---|---|---|
1 | 1.59 | 0.994 |
2 | 11.1 | 6.94 |
3 | 9.54 | 5.96 |
4 | 15.9 | 9.94 |
5 | 6.36 | 3.98 |
Millikan's results showed that the charge on each oil drop was an integer multiple of 1.60 × 10−19 C
Quantisation of Electric Charge
Each negative oil droplet had been ionised, so carried an integer number of electrons
The fact that each droplet's charge was quantised into integer multiples of 1.60 × 10−19 C suggested that this was the charge of each electron
Worked Example
Using Stokes' Law, show that the droplet's mass m can be expressed in terms of its velocity v, the viscosity of air η, the density of the oil ρ and acceleration due to gravity g, as:
Answer:
Step 1: Write down the relevant equation from the data and formulae sheet
Stokes' law:
Step 2: Apply Stokes' law to the droplet at terminal velocity
At terminal velocity (in the absence of an electric field), weight is equal to viscous drag force:
Step 3: Write mass in terms of radius and density
We need to get rid of the radius term as it will not be in our final expression
We can do this by writing radius in terms of the required quantities
Recall the equation relating mass, density and volume of a sphere, Vs :
Step 4: Write an expression for the droplet's radius
Substitute the expression for mass in terms of radius and density and rearrange for radius:
Step 5: Substitute this into the expression for mass
We can finally eliminate radius from the equation in step 2:
Examiner Tips and Tricks
"Show that" questions are one of the rare moments in an exam you can be totally confident you have the correct answer, but don't get complacent. The marks are awarded for clear and logical working out. Don't try to skip a step and hope you can convince your examiner, be thorough.

Unlock more, it's free!
Did this page help you?