Thermodynamic Processes (AQA A Level Physics): Revision Note
Exam code: 7408
Thermodynamic Processes
The four main thermodynamic processes are
constant volume
constant pressure
isothermal
adiabatic
Constant pressure
An isobaric (constant pressure) process is defined as:
A process in which no change in pressure occurs
This occurs when gases are allowed to expand or contract freely during a change in temperature
When there is a change in volume ΔV at a constant pressure p, work done W is equal to
From the first law of thermodynamics:
The ± sign reflects whether work has been done on or by the gas as a result of the change in volume
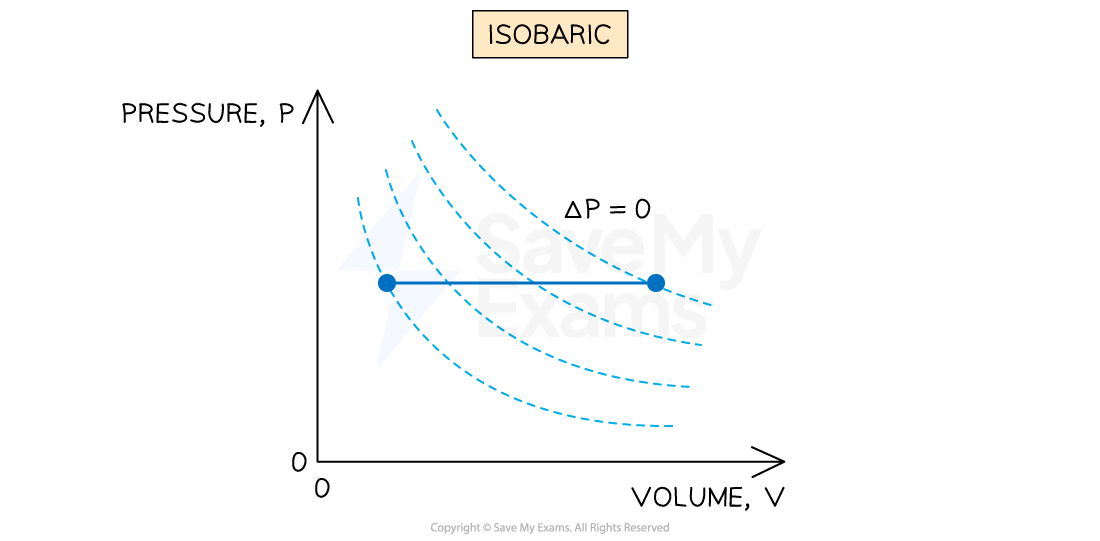
The solid blue line represents an isobaric process at constant pressure on a p-V diagram
Constant volume
An isovolumetric (constant volume) process is defined as:
A process where no change in volume occurs and the system does no work
If there is no change in volume, then there is no work done on or by the gas, so
Therefore, from the first law of thermodynamics:
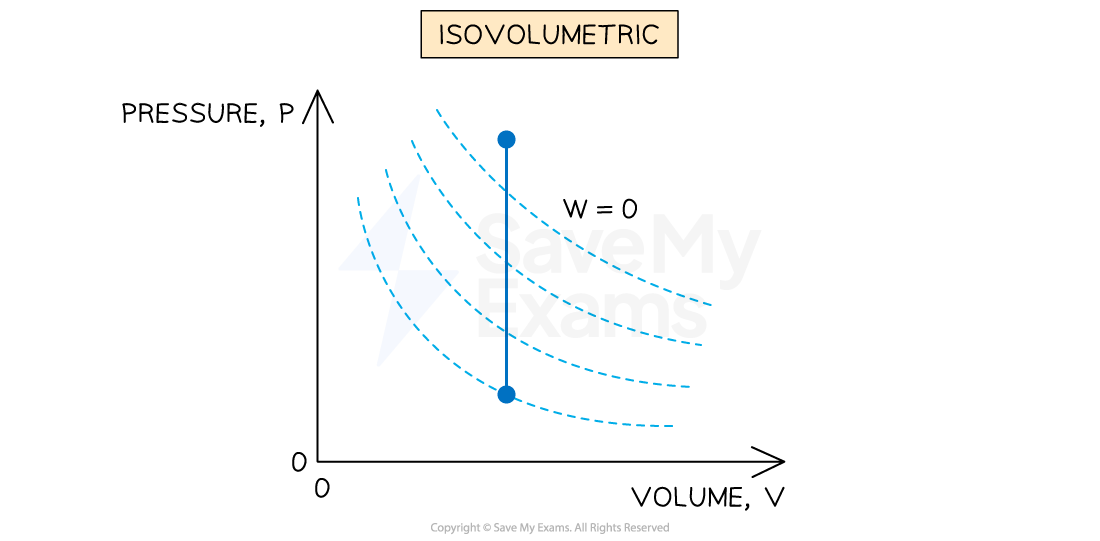
The solid blue line represents an isovolumetric process at constant volume on a p-V diagram
Constant temperature (isothermal)
An isothermal process is defined as:
A process in which no change in temperature occurs
If the temperature does not change, then the internal energy of the gas will not change, so
Therefore, from the first law of thermodynamics:
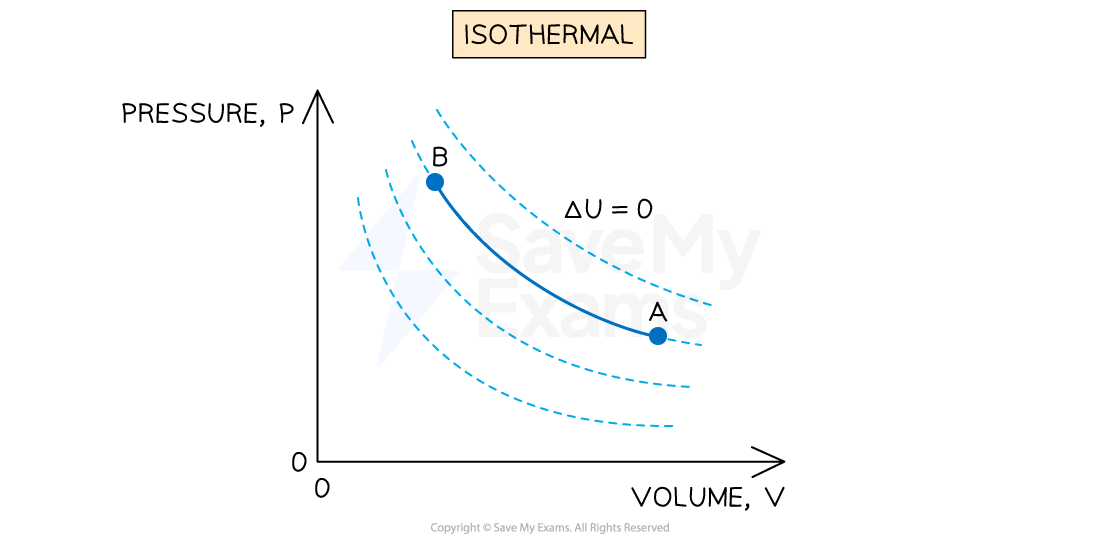
The solid blue line represents an isothermal process with constant temperature on a p-V diagram
Constant thermal energy (adiabatic)
An adiabatic process is defined as:
A process where no heat is transferred into or out of the system
If there is no heat entering or leaving the system then
Therefore, from the first law of thermodynamics:
This means that all the work done is at the expense of the system's internal energy
Hence, an adiabatic process will usually be accompanied by a change in temperature
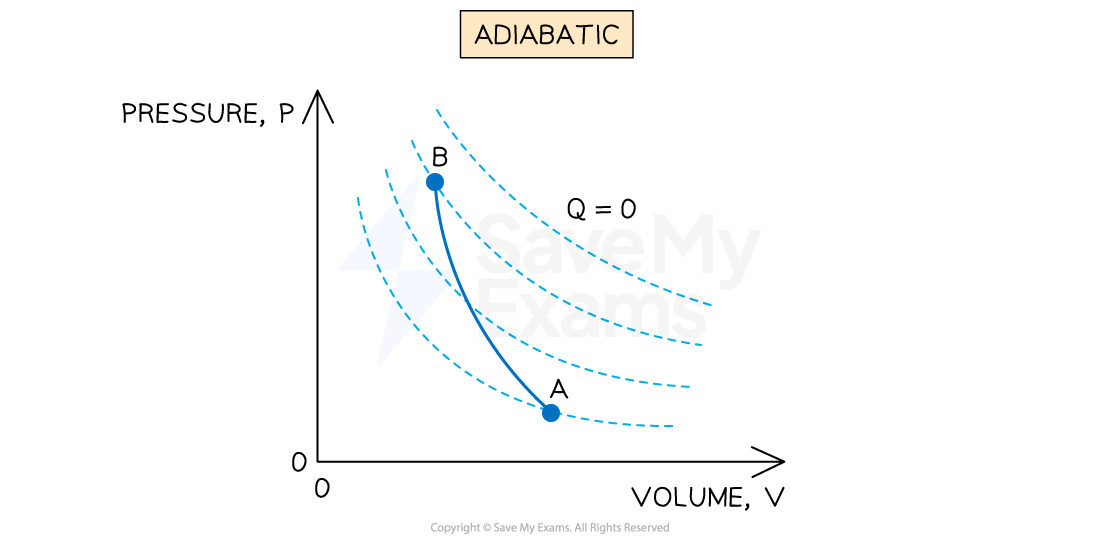
The solid blue line represents an adiabatic process with constant thermal energy on a p-V diagram
Adiabatic Processes
Adiabatic processes in ideal gases can be modelled by the equation
Where:
p = pressure of the gas (Pa)
V = volume occupied by the gas (m3)
This equation can be used for calculating changes in pressure, volume and temperature, e.g. for monatomic ideal gases, where
Where:
= initial pressure (Pa)
= final pressure (Pa)
= initial volume (m3)
= final volume (m3)
Worked Example
A quantity of energy Q is supplied to three ideal gases X, Y and Z.
Gas X absorbs Q isothermally, gas Y isovolumetrically and gas Z isobarically.
Complete the table by inserting the words ‘positive’, ‘zero’ or ‘negative’ for the work done W, the change in internal energy ΔU and the temperature change ΔT for each gas.
| |||
|---|---|---|---|
X |
|
|
|
Y |
|
|
|
Z |
|
|
|
Answer:
X: Isothermal = constant temperature, no change in internal energy
Temperature:
Internal energy:
, so,
Work done:
Y: Isovolumetric = constant volume, no work done
Work done:
, so,
Internal energy:
Temperature:
, so,
Z: Isobaric = constant pressure
Work done:
, so
, so
Internal energy:
, so
Temperature:
, so
| |||
|---|---|---|---|
X | positive | 0 | 0 |
Y | 0 | positive | positive |
Z | positive | positive | positive |
Worked Example
A heat engine operates on the cycle shown in the pressure-volume diagram. One step in the cycle consists of an isothermal expansion of an ideal gas from state A of volume V to state B of volume 2V.
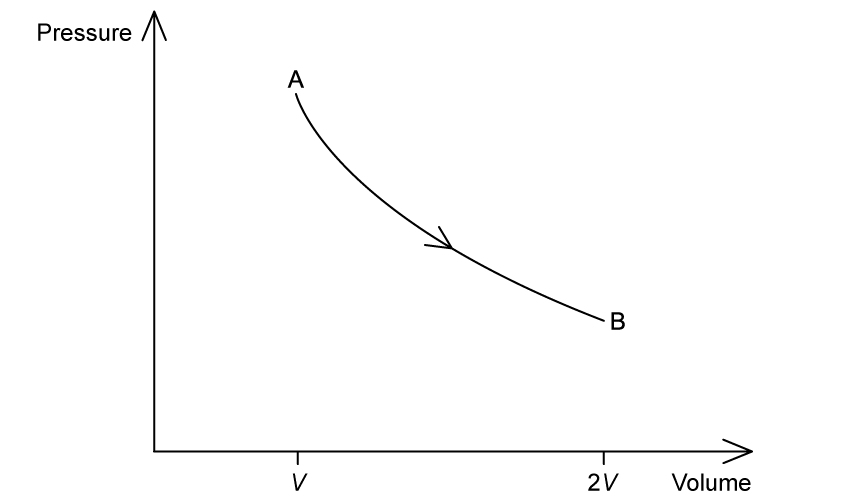
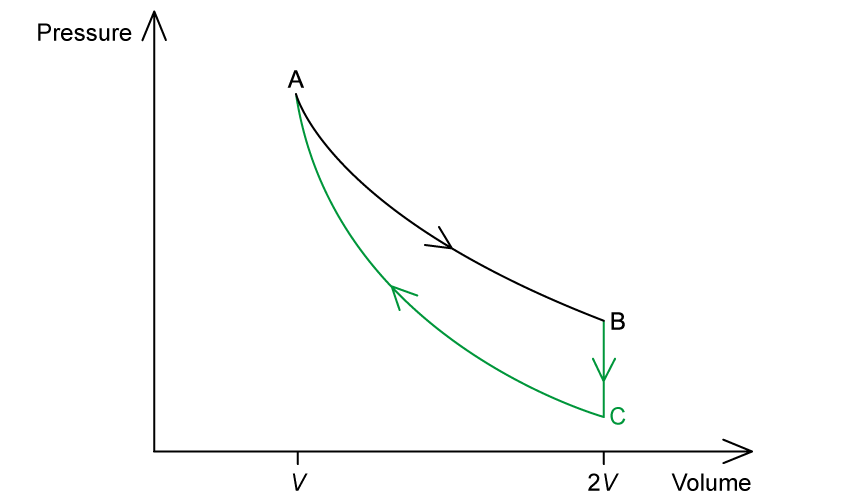
On the graph, complete the cycle ABCA by drawing curves to show
a change at constant volume from state B to state C
an adiabatic compression from state C to state A
Answer:
Constant volume = no work done
Next step is a compression (where pressure increases), so this step should involve a pressure drop
Hence, B to C: line drawn vertically down
Adiabatic = no heat supplied or removed, compression = work is done on the gas, volume decreases
Hence, C to A: line curves up to meet A
Worked Example
An ideal monatomic gas expands adiabatically from a state with pressure 7.5 × 105 Pa and volume 1.8 × 10−3 m3 to a state of volume 4.2 × 10−3 m3.
Calculate the new pressure of the gas.
Answer:
For an ideal monatomic gas undergoing an adiabatic change:
Where:
Initial pressure,
= 7.5 × 105 Pa
Final pressure =
Initial volume,
= 1.8 × 10−3 m3
Final volume,
= 4.2 × 10−3 m3
New pressure: = 1.8 × 105 Pa

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?