Heat Engines (AQA A Level Physics): Revision Note
Exam code: 7408
Efficiency of a Heat Engine
The goal of a heat engine is to transfer thermal energy (heat) into useful mechanical work as efficiently as possible
The efficiency of a heat engine can, therefore, be calculated using
Where:
W = useful work output (J)
QH = energy transferred from the source (J)
QC = energy transferred to the sink (J)
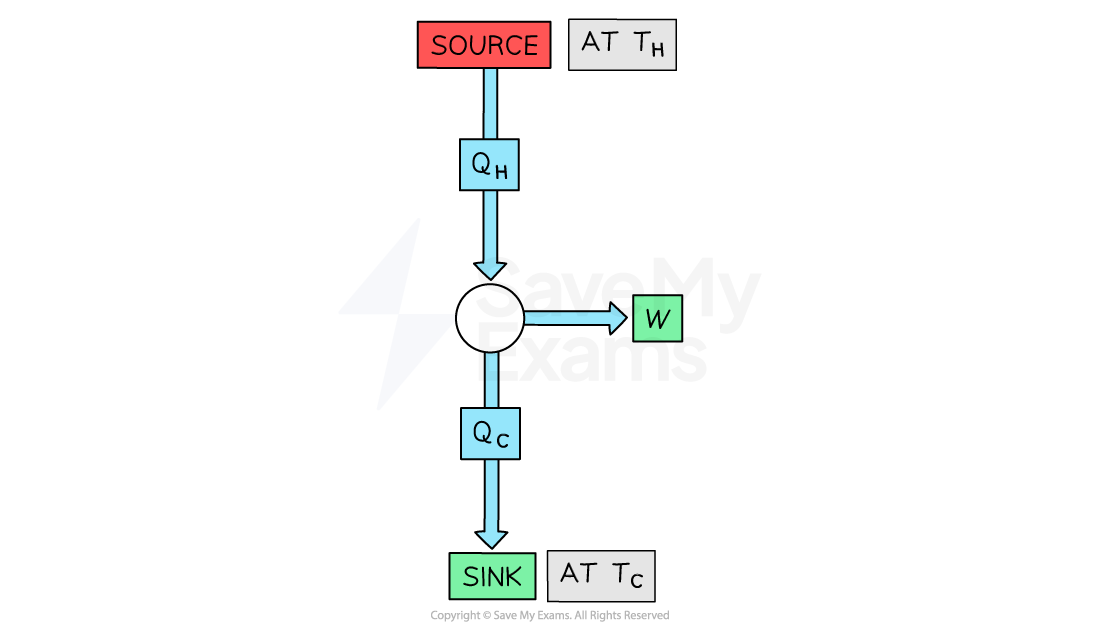
Source-sink diagram for a heat engine
For the efficiency of a heat engine to be 0%, this would mean that no useful work is done
Conversely, the efficiency of a heat engine can never be 100%, as no heat engine can completely convert heat into work
This is because some heat will always be lost to the surroundings
Worked Example
Which is a correct statement about an ideal heat engine?
A. The efficiency is 75% when the absolute temperature of the hot source is four times the absolute temperature of the cold sink.
B. The maximum efficiency depends on the p-V cycle of the engine.
C. The efficiency decreases when the absolute temperature of the hot source and the cold sink decrease by equal amounts.
D. The efficiency is 25% when the absolute temperature of the hot source is four times the absolute temperature of the cold sink.
Answer: A
If the hot source is four times the absolute (Kelvin) temperature of the cold sink, then
In the efficiency equation, this is
This efficiency is 75 %
Examiner Tips and Tricks
This equation is given on your data sheet. Make sure all the variables are in the same units i.e. J or kJ. You must convert these into the same units before you do your calculation.
If the efficiency is asked for as a percentage instead of a decimal, remember to × 100
Maximum Theoretical Efficiency
As the efficiency of a thermodynamic system increases, the difference between the temperatures of the source and sink increases
The maximum theoretical efficiency of a heat engine is:
Maximum theoretical efficiency =
Where:
= temperature in the sink (cold reservoir) (K)
= temperature in the source (hot reservoir) (K)
This equation can be used if an ideal gas is used as a substance for the engine
Therefore, to make an engine as efficient as possible, the source temperature must be as high as possible, and the sink temperature as low as possible
The maximum theoretical efficiency is 100% only if the sink temperature is at 0 K
Worked Example
An engineer designs a heat engine that has an inlet temperature of 500 K and an outlet temperature of 300 K. The engineer claims that 100 kJ of thermal energy flows out of the source and 25 kJ of thermal energy flows into the sink.
Determine, with reference to the second law of thermodynamics, whether this engine is thermodynamically possible.
Answer:
Step 1: Determine the efficiency of the proposed engine
The efficiency of this engine would be
Where:
Heat transferred in,
= 100 kJ
Heat transferred out,
= 25 kJ
Efficiency = = 0.75 = 75%
Step 2: Determine the maximum theoretical efficiency of the proposed engine
A Carnot engine operating between the same temperatures would have an efficiency of
Maximum theoretical efficiency =
Where:
Inlet temperature,
= 500 K
Outlet temperature,
= 300 K
Maximum theoretical efficiency = = 0.4 = 40%
Step 3: Discuss the proposed engine in relation to the second law
The second law of thermodynamics states that it is impossible for heat to flow from a cooler body to a hotter body without performing work
This law sets an upper limit on the maximum possible efficiency of the transfer of thermal energy to mechanical energy in a heat engine
The maximum possible efficiency of the proposed engine is 40%, but the engineer is proposing an efficiency of 75% i.e. an efficiency greater than the efficiency of the engine
This violates the second law, hence the proposed engine is impossible
Examiner Tips and Tricks
This equation is valid for all idealised reversible engines, irrespective of the particular cycle and the particular working substance. You may be given unfamiliar cycles in the exam (such as the Carnot cycle) to apply this to.

Unlock more, it's free!
Did this page help you?