This question is about thin layer chromatography.
Name the two phases of chromatography and give one example of a chemical used for each phase.
State three methods or chemicals that can be used to locate or make the spot of a non-coloured compound visible.
Thin layer chromatography is used to check if an unknown food colouring contains a banned colouring.
Draw a labelled diagram to show the experimental setup for this TLC analysis.
State two factors that the rate of separation depends upon.
State the equation used to calculate the unique retention factor of a compound.
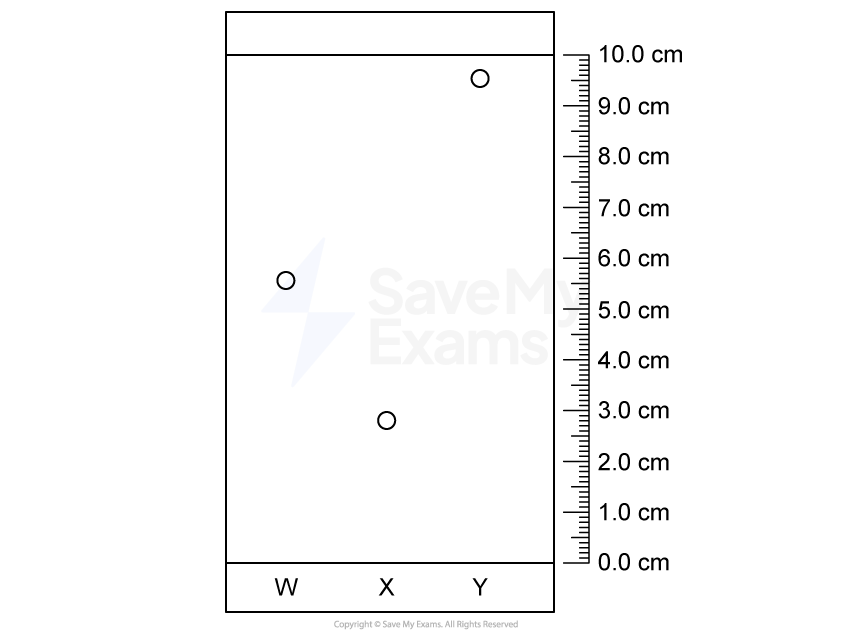
Calculate the Rf value of the compound shown in the chromatogram below.

Was this exam question helpful?